Ali Sharif Moqadam1, Valiollah Arash1, Maysam Mirzaie1, Majid Fereydooni2, Hamid Haghani3 and Aghil Rahmani1
1Department of Orthodontics, School of Dentistry, Babol University of Medical Sciences, Babol, Iran.
2Department of Periodontology, School of Dentistry, Babol University of Medical Sciences, Babol, Iran.
3Department of Biostatistics, School of Public Health, Iran University of Medical Sciences, Tehran, Iran.
Corresponding Author E-mail: vali_arash1344@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1072
Abstract
Tooth extraction results in resorptive remodeling of the alveolar bone,but alveolar ridge preservationprocedure maintains the original shape of the extraction socket. This may be beneficial for space closure by orthodontic tooth movement (OTM). In the current pilot study forrandomized controlled clinical trial the effect of alveolar ridge preservationwith partial demineralized freeze-dried bone allograft (PDFDBA) on OTM rate, formation of gingival invagination and root resorptionwas evaluated. Both mandibular first premolars of6 patients were extracted due to orthodontic treatment. In a split-mouth study design,alveolar ridge preservation was performed on one side, while the other side served as a control and the extraction socket healed naturally. After 6 weeks of healing period, the canines were moved to the extraction siteto close the extraction space. Eight weeks later, the amount of OTM was measured. After space closure, the extraction sites were examined for the presence of gingival invagination. Root resorption was evaluated on digital panoramic radiographs. Photographs were taken for documentation. There was no significant difference in OTM rate betweenthe ridge preserved areas and naturally healed sockets. Gingival invagination formed in 5 of 6 naturally healed sockets; none of the ridge preserved areas showed formation of gingival invagination. No root resorption was observed in any of the teeth adjacent to the extraction sites. Alveolar ridge preservation with PDFDBA has no effect on the rate of OTM and root resorptionbut preventsformation of gingival invagination during orthodontic space closure.
Keywords
socket graft; allograft; tooth movement; root resorption
Download this article as:| Copy the following to cite this article: Moqadam A. S, Arash V, Mirzaie M, Fereydooni M, Haghani H, Rahmani A. Effect of Alveolar Ridge Preservation with PDFDBA on Orthodontic Tooth Movement Rate, Formation of Gingival Invagination and Root Resorption: A Randomized, Controlled Pilot Study. Biomed Pharmacol J 2016;9(3). |
| Copy the following to cite this URL: Moqadam A. S, Arash V, Mirzaie M, Fereydooni M, Haghani H, Rahmani A. Effect of Alveolar Ridge Preservation with PDFDBA on Orthodontic Tooth Movement Rate, Formation of Gingival Invagination and Root Resorption: A Randomized, Controlled Pilot Study. Biomed Pharmacol J 2016;9(3). Available from: http://biomedpharmajournal.org/?p=11879 |
Introduction
Tooth extraction is a common method of providing space for orthodontic treatments. Caries, periodontal diseases and traumas may also lead to tooth extraction. Consequences of these extractions areresorptive remodeling of the alveolar bone[1,2], especially in the coronal segment of buccal bone plate[3],a decrease in the volume of the alveolar bone and unfavorable architecture of the residual alveolar bone. This may lead to problematic space closure by orthodontic tooth movement (OTM)[4].
Gingival invagination is a cleft in the interproximal tissue with vertical and horizontal probing depth of at least 1 mm, occurring following space closure in extraction sites[5]. This happens in 35‒100% of patients[6]. Consequences of this gingival infoldingare increased marginal bone loss in the interproximal space, impaired patient’s oral hygiene ability and increased time need for space closure. Even sometimes it is impossible to completely close the space due to gingival tissue hyperplasia. The stability of treatment results may also be at risk[6]. It has been shown that preserving the extraction socket with a non-resorbable membrane inhibited formation of gingival invagination[7].
Alveolar ridge preservation is a surgical technique to reduce bone loss after tooth extraction to keep the socket in its original shape. This technique maintains the composition of regenerated woven bone, which is considered the favorable matrix for tooth movement and results in easier and less detrimental OTM[8].
A variety of materials from different sources are available for ridge preservation, which consist of autogenous bone, allografts, xenografts and alloplasts[9]. Although autogenous bone is the best bone graft because of the vitality of osteogenic cells, it requires another surgery in a donor site and many patients refuse this treatment because of the additional discomfort in the donor site. For this reason many surgeons prefer to use bone substitutes[10].Freeze-dried bone allografts (FDBA) and demineralized freeze-dried bone allografts (DFDBA) are the most common allografts used for alveolar ridge preservation[11].DFDBA is both osteoinductive and osteoconductive, while FDBA is only osteoconductive; however, it has more osteoconductive effect than DFDBA does[11,12].FDBA induced more bone regeneration when compared with DFDBA[13], but DFDBA showed more vital bone 19 weeks after placement in the extraction socket[14].
Partial demineralized freeze-dried bone allograft (PDFDBA)(CenoBone®) is a new bone substitute product, which consist of 70% FDBA and 30% DFDBA; therefore, it has properties of both DFDBA and FDBA.
Although many studies have shown favorable effects of moving teeth into the bone graft[8,15-18], several studies have shown adverse effects of such movement in bone grafts, such as root resorption and stagnation of tooth movement[19].
The aim of this pilot study forrandomized controlled clinical trialwas to evaluate the effect of alveolar ridge preservation with PDFDBA on orthodontic tooth movement rate, formation of gingival invagination and amount of root resorption.
Materials and Methods
The trial was registered on the IranianRegistry of Clinical Trials under the registration code IRCT2015091524039N1.
Before the study, all the procedures were approved by the Ethics Committee of Babol University of Medical Sciences (under the code MUBABOL.REC.1394.182).
Six patients with an age range of 14‒20 years and no remarkable medical history or specific diseases, who were in need of extraction of analogous mandibular first premolars due to space deficiency or dentoalveolar protrusion, were selected for this clinical trial with split-mouth randomization. All the canines and second premolars were within the dental arch and had fully developed and straight roots. Patients who had periodontal disease were excluded from the study.The study procedures were explained to the patients and their parents and written consent forms were taken from them prior to the study.
The first molars were banded and stainless steel preadjusted edgewise bracketswith an 0.022-inch slot size (MBT, American Orthodontics, Sheboygan, WI,USA) were bonded to all the teeth mesial to the first molars.Initial aligning and leveling was performed using Ni-Ti wires. Incisors which did not have enough space for aligning were not engaged with wireat thistreatment phase.
Analogous first premolars on each side were extracted atraumatically using forceps and elevators (Fig 1, A). On the basis of split-mouth study design,by using a coin randomization extraction sockets in one side were filled with PDFDBA powder (CenoBone® 150-2000 μm, Tissue Regeneration Corporation, Kish, Iran) (Fig 1, B), covered with collagen membrane (CenoMembrane® 0.4‒0.6 mm, Tissue Regeneration Corporation, Kish, Iran)(Fig 1,C) and finally stitched with 3 single interrupted sutures (Supasil® 3/0 USP, Supa, Tehran, Iran) (Fig 1, D). The other side served as a control area and only 3 single interrupted sutures were applied to stabilize the blood clot in the extraction socket. Two weeks later, the sutures were removed. In order to avoid infection in the surgical sites, during this 2-week period after surgery the patients were asked to use0.12% chlorhexidinegluconatemouthwashtwicedaily.Following extraction and ridge preservation, the wounds were allowed to heal for 6 weeks. After the healing phase,the distance between the canine and second premolar on each side (initial distance) was measured bylocating the sharp tips of digital caliper (Mitutoyo, Kawasaki, Japan) accurateto 0.01 mm on the cementoenamel junction (CEJ) of theseteeth.Then space closure was carried out on 0.018×0.025-inch stainless steel wire by Ni-Ti closed coil spring (12 mm, medium, Ortho Technology®, Tampa, FL,USA) placed between the canine and first molar (Fig 2). When activated, this spring delivers a constant pulling force of 150 g, which is believed to be the optimum force for canine retraction[20].
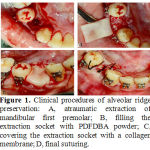 |
Figure 1: Clinical procedures of alveolar ridge preservation: A, atraumatic extraction of mandibular first premolar; B, filling the extraction socket with PDFDBA powder; C, covering the extraction socket with a collagen membrane; D, final suturing. |
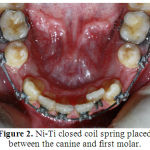 |
Figure 2: Ni-Ti closed coil spring placed between the canine and first molar. |
After 8 weeks, the distance between the canine and second premolarwas measured again like the previous time (the final distance) and the exact amount of OTM was obtained by subtracting the final distance from the initial distance.
When space closure was completed, interdental gingiva between the canine and first premolarwasexamined by a universal periodontal probe for the presence of gingival invagination (Fig 3). A digital panoramic radiograph was taken (final panoramic) and compared with the pretreatment digital panoramic view (initial panoramic) to evaluate root resorption of the teeth adjacent to the extraction space. On each session of treatment photographs were taken for documentation.
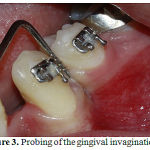 |
Figure 3: Probing of the gingival invagination |
SPSS 15 was used for statistical analysis. Wilcoxon test was used for comparison of OTMand Fisher’s exact test was used for comparison of gingival invagination between the ridge-preserved and control sites.
Results
There was an equal amount of OTM in ridge-preserved and naturally healed areas in one patient and there was a little difference in other patients (Table 1). In two patients, there was more OTM in ridge-preserved areas and in 3 patients OTM was higher in naturally healed sockets (Fig 4). Overall, there was no statistically significant difference in the amount of OTM between the two groups (P=0.674).
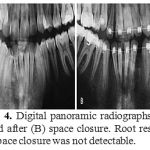 |
Figure 4: Digital panoramic radiographs before (A) and after (B) space closure. Root resorption after space closure was not detectable. |
Table 1: Means and standard deviations of initial and final distance and amount of OTM (mm) in ridge preserved and naturally healed areas
| ridge preserved areas | naturally healed areas | P value | |
| Initial distance | 7.26 ± 0.67 | 7.17 ± 0.55 | 0.753 |
| Final distance | 4.22 ± .078 | 4.25 ± 0.41 | 0.753 |
| Variations (OTM) | 3.04 ± .041 | 2.92 ± 0.77 | 0.674 |
Wilcoxon test was performed
After complete space closure, gingival invagination was observed in 5 of 6 (83.3%) naturally healed sockets, but none of the ridge-preserved areas showed formation of gingival invagination. This difference was statistically significant(P=0.015).
When final panoramic views were compared with initial panoramic views, no signs of root resorption could be observed in teeth adjacent to the extraction sites, neither in ridge-preservednor in naturally healedareas (Fig 5).
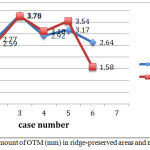 |
Figure 5: Graph of the amount of OTM (mm) in ridge-preserved areas and naturally healed sockets. |
Discussion
The results of the present study showed that alveolar ridge preservationwith PDFDBA prevents formation of gingival invagination after moving the tooth into the extraction space. However, in the majority of cases in which the extraction socket healed naturally without ridge preservation procedure, after the space was closed by tooth movement, there was gingival invagination. Reichert et al[16] and Tiefengarber et al[7] reported similar results. Since different materials were used for ridge preservation in these studies and in the present study, it can be claimed that the ridge preservation procedure itself, not the graft material, is important in inducing gingival invagination. Even in the study by Tiefengarber et al no bone graft was used and ridge preservation was carried out only by covering the tooth socket with a membrane[7]. This can be explained by the fact that the ridge preservation procedure results in maintaining the integrity and width of the alveolar ridge; therefore, no empty space will exist to give rise to gingival soft tissue infolding into the alveolar ridge during the space closure. However, in tooth extraction without ridge preservation procedure, natural remodeling of the alveolar ridge, which consists of resorption, will result in a decrease in the width of the alveolar bone and gingival infolding into this space, forming gingival invagination.
Another finding of the present study was the fact that tooth movement rate within the bone graft resulting from the ridge preservation technique was not significantly different from that within the remodeled bone resulting from natural healing after tooth extraction. In studies by Reichert et al[16], Tiefengarber et al [7]and Sheats et al[21], similar results were achieved; in these studies, too, similar to the present study, the surgical sites were allowed to heal for at least 6 weeks before application of force. However, Sefi et al[18] and Kim et al [8]carried out animal studies in which they began moving the teeth immediately after surgery, without giving the wound time to heal and consolidate; they showed that the tooth movement rate was higher within the bony graft. This can be attributed to the time needed for consolidation of the graft after surgery and before initiation of force application. After ridge preservation, the graft material resorbs over time during the healing and consolidation process and woven bone, which is the best bone for tooth movement and is preserved by the bone graft, is gradually converted into lamellar bone and moving the teeth is difficult within this type of bone[8]. The consequence is a decrease in the speed of tooth movement in the graft area. However, in human subjects, before moving the teeth within the graft, there is need for graft consolidation and tissue regeneration before moving the tooth into the graft; several studies have recommended at least 3‒6 weeks of time[8].
On the other hand, Ru et al showed that the tooth movement rate in defects grafted with BoneCeramic was less than that in defects that healed naturally without any grafts[17], which might be attributed to the composition of BoneCeramic, consisting of a high percentage (60%) of hydroxyapatite and since this material is completely mineralized, it requires a long time for resorption. Therefore, a decrease in tooth movement rate in this mineralized dense matrix is expected.
Follow-up examinations in the present study showed that in 4 cases of 5 areas in which gingival invagination had occurred, the tooth movement rate had decreased at the end of space closure period. Reichert et al [16]and Wehrebin et al[22], too, showed this effect of gingival invagination in delaying the space closure at the end of treatment, which might be attributed to condensation during the epithelial and connective tissue proliferation of the gingival invagination, resulting in the application of a force to teeth in a direction opposite to the direction of space closure. This might explain the re-opening of space and relapse of treatment results after complete closure of the space.
In the present study, digital panoramic radiography evaluations before and after space closure showed no root resorption in any of the cases. Reichert et al[16], too, after space closure in the area with and without bone substitute, showed no root resorption on periapical and panoramic radiographs. However, Schneideret al [23] showed cessation of movement and root resorption during tooth movement within the hydroxyapatite ceramicalloplast, which might be attributed to the very slow resorption of this mineral material. On the other hand, Seifi et al[18], Oltramari et al [15]and Ru et al [17]showed in animal studies that tooth movement within the bone graft results in a decrease in root resorption compared to the time when the tooth is moved within the bone resulting from natural healing of the extraction socket. This might be attributed to the use of histological and CBCT radiographic techniques for the evaluation of root resorption in the studies above, which are much more accurate than two-dimensional periapical and panoramic radiographic evaluations. In addition, minor apical resorption that is not detectable on panoramic radiographs is usually not significant. Finally, it should be pointed out that since the present study was carried out on human subjects, it was not possible to carry out histological evaluations. In addition, administration of a high dose of CBCT technique for radiographic evaluations is not ethically acceptable in the case of human subjects.
Conclusion
Alveolar ridge preservationafter tooth extraction hinders formation of gingival invagination during orthodontic space closure. After a 6-week healing phase following alveolar ridge preservationwith PDFDBA, there was no difference in OTM rate in ridge-preserved areas and naturally healed sockets but the final space closure might take more time in naturally healed sockets due to the formation of gingival invagination. Alveolarridge preservation with PDFDBA had no effect on root resorption of teeth which has been moved into the extraction space.
Acknowledgement
We would like to thankShimaHaghani for kindly participating in the statistical analyses and Tissue Regeneration Corporation for providing PDFDBA and collagen membrane.
References
- Abrams H, Kopczyk RA, Kaplan AL. Incidence of anterior ridge deformities in partially edentulous patients. J Prosthet Dent. 1987 Feb;57 (2):191-194.
- Atwood DA, Coy WA. Clinical, cephalometric, and densitometric study of reduction of residual ridges. J Prosthet Dent. 1971 Sep;26 (3):280-295.
- Araujo MG, Sukekava F, Wennstrom JL, Lindhe J. Ridge alterations following implant placement in fresh extraction sockets: an experimental study in the dog. J Clin Periodontol. 2005 Jun;32 (6):645-652.
- Diedrich PR. Guided tissue regeneration associated with orthodontic therapy. Semin Orthod. 1996 Mar;2 (1):39-45.
- Golz L, Reichert C, Dirk C, Jager A. Retrospective investigation of gingival invaginations: Part II: microbiological findings and genetic risk profile. J Orofac Orthop. 2012 Sep;73 (5):387-396.
- Golz L, Reichert C, Jager A. Gingival invagination–a systematic review. J Orofac Orthop. 2011 Nov;72 (6):409-420.
- Tiefengraber J, Diedrich P, Fritz U, Lantos P. Orthodontic space closure in combination with membrane supported healing of extraction sockets (MHE) a pilot study. J Orofac Orthop. 2002 Sep;63 (5):422-428.
- Kim KA, Choi EK, Ohe JY, Ahn HW, Kim SJ. Effect of low-level laser therapy on orthodontic tooth movement into bone-grafted alveolar defects. Am J Orthod Dentofacial Orthop. 2015 Oct;148 (4):608-617.
- Jamjoom A, Cohen RE. Grafts for Ridge Preservation. J Funct Biomater. 2015 Aug;6 (3):833-848.
- Cardaropoli G, Araujo M, Hayacibara R, Sukekava F, Lindhe J. Healing of extraction sockets and surgically produced – augmented and non-augmented – defects in the alveolar ridge. An experimental study in the dog. J Clin Periodontol. 2005 May;32 (5):435-440.
- AlGhamdi AS, Shibly O, Ciancio SG. Osseous grafting part I: autografts and allografts for periodontal regeneration–a literature review. J Int Acad Periodontol. 2101 Apr;12 (2):34-38.
- Piattelli A, Scarano A, Corigliano M, Piattelli M. Comparison of bone regeneration with the use of mineralized and demineralized freeze-dried bone allografts: a histological and histochemical study in man. Biomaterials. 1996 Jun;17 (11):1127-1131.
- Yukna RA, Vastardis S. Comparative evaluation of decalcified and non-decalcified freeze-dried bone allografts in rhesus monkeys. I. Histologic findings. J Periodontol. 2005 Jan;76 (1):57-65.
- Wood RA, Mealey BL. Histologic comparison of healing after tooth extraction with ridge preservation using mineralized versus demineralized freeze-dried bone allograft. J Periodontol.2012 Mar;83 (3):329-336.
- Oltramari PV, de Lima Navarro R, Henriques JF, Taga R, Cestari TM, Ceolin DS, et al. Orthodontic movement in bone defects filled with xenogenic graft: an experimental study in minipigs. Am J Orthod Dentofacial Orthop. 2007 Mar;(3):302.e310-307.
- Reichert C, Wenghofer M, Gotz W, Jager A. Pilot study on orthodontic space closure after guided bone regeneration. J Orofac Orthop. 2011 Mar;72 (1):45-50.
- Ru N, Liu SS, Bai Y, Li S, Liu Y, Wei X. BoneCeramic graft regenerates alveolar defects but slows orthodontic tooth movement with less root resorption. Am J Orthod Dentofacial Orthop. 2016 Apr;149 (4):523-532.
- Seifi M, Ghoraishian SA. Determination of orthodontic tooth movement and tissue reaction following demineralized freeze-dried bone allograft grafting intervention. Dent Res J (Isfahan). 2012 Mar;9 (2):203-208.
- Reichert C, Gotz W, Smeets R, Wenghofer M, Jager A. The impact of nonautogenous bone graft on orthodontic treatment. Quintessence Int. 2010 Sep;41 (8):665-672.
- Miura F, Mogi M, Ohura Y, Hamanaka H. The super-elastic property of the Japanese NiTi alloy wire for use in orthodontics. Am J Orthod Dentofacial Orthop. 1986 Jul;90 (1):1-10.
- Sheats RD, Strauss RA, Rubenstein LK. Effect of a resorbable bone graft material on orthodontic tooth movement through surgical defects in the cat mandible. J Oral Maxillofac Surg. 1991 Dec;49 (12):1299-1303; discussion 1304.
- Wehrbein H, Fuhrmann R, Andreas A, Diedrich P. Die Bedeutung von Gingivaduplikaturen beim orthodontischen Lückenschluß. Eine klinisch-radiologischeStudie. Fortschr Kieferorthop. 1993;54:231-236
- Schneider B, Diedrich P. Interaktion von kieferorthopädischer Zahnbewegung und Hydroxylapatit-Keramik. Dtsch Zahnärztl. 1989;44:282-285.








