Bui Thi Binh1, Tran Thi Hien1,2, Do Thi Ha3, Pham Duc Chinh4, Le Viet Dung3 and Nguyen Thi Bich Thu3
1Thaibinh Medical University.
2 Departments of Experimental Medical Sciences, Faculty of medicine, Lund University, Sweden.
3National Institute of Medicinal Materials, 3B Quangtrung, Hanoi.
4Thai Nguyen University of Agriculture and Forestry, Quyet Thang, Thai Nguyen, Vietnam.
Corresponding Author E-mail: thi.hien_tran@med.lu.se
DOI : https://dx.doi.org/10.13005/bpj/1029
Abstract
Bioassay-guided fractionation of the EtOAc soluble fraction from the rhizomes of Belamcanda chinensis resulted in the isolation of eleven compounds (BC-1 to BC-11), those were evaluated for COX-2 expression and prostaglandin E2 (PGE2) production in Raw264.7 cells. Among them, BC-2 ((7R,8S)-dehydrodiconiferyl alcohol-9′γ-methyl ether) showed the most potent inhibitory activities by suppressing LPS-induced COX-2 expression and PGE2 production in a dose-dependent manner. Due to increasing evidence for the role of microRNAs (miRNAs) in the regulation of inflammation diseases, we examined the effects of BC-2 with or without LPS on miR-146a-5p, miR-146a-3p, miR-155, miR-25 and miR-147. As the results, they were significantly induced by LPS treatment, but only miR-146a-5p and miR-155 were reduced in the presence of BC-2. Furthermore, miR-146a-5p mimic and miR-155 mimic increased COX-2 protein and mRNA expression. However, these increases were abolished with the treatment of BC-2. These data indicate that the anti-inflammatory action of BC-2 in Raw246.7 cells involves in miR-146a and miR-155, and might enable the design of novel therapeutic agents of inflammatory diseases in the future.
Keywords
Belamcanda chinensis; (7R,8S)-dehydrodiconiferyl alcohol-9′γ-methyl ether; COX-2; PGE2; miR-146a; miR-155
Download this article as:| Copy the following to cite this article: Binh B. T, Hien T. T, Ha D. T, Chinh P. D, Dung L. V, Thu N. T. B. Anti-Inflammatory Effect of (7R,8S)-Dehydrodiconiferyl Alcohol-9′γ-Methyl Ether from the Rhizome of Belamcanda Chinensis: Role of Mir-146a and Mir-155. Biomed Pharmacol J 2016;9(3). |
| Copy the following to cite this URL: Babaloo A, Rahbar M, Ghasemi S, Shirmohammadi A, Dibaj A. Attitudes of Students Toward Clinical Education and Evaluations Made in the Department of Periodontics in Tabriz Faculty of Dentistry in 2015. Biomed Pharmacol J 2016;9(3). Available from: http://biomedpharmajournal.org/?p=11934 |
Introduction
The cyclooxygenase (COX) including COX-1 and COX-2 isoenzymes, act as important role of inflammatory response and a formation of prostanoids including prostacyclin, prostagalandins and thromboxane(1). COX-1 is known to be present in most tissues and involves in maintaining tissue homeostasis and cell signaling. Also, COX-1 is shown in angiogenesis in endothelial cells (2). COX-2 is an important key enzyme and an inducible form that catalyze in the biosynthesis of prostaglandins. COX-2 is well-know gene associated with inflammatory mediator participated in numerous of biological processes such as: pain, inflammation, cancer, angiogenesis, carcinogenesis or development of immunity (3).
Non-steroidal anti-inflammatory drugs (NSAIDs) and corticosteroids are main therapeutics to reduce both pain and inflammation. Unfortunately, they have side effects especially corticosteroids when used for long periods, including diabetes, osteoporosis, high blood pressure, cataracts, and an increased risk of infection. Thus, plant-derived compounds are gaining increasing interest as potential anti-inflammation therapeutics, particularly for treatment-chronic inflammation. Some natural compounds in fruits, vegetables, and herbs suppressed inflammatory activity were showed.[6,7] Especially, those were found in traditional medicine and dietary supplements have the potential to be developed as COX-2 inhibitor.
Belamcanda chinensis (L.) DC (Iridaceae) is a perennial shrub, 0.3–0.8 m in length, which is distributed in the cold and wet hillsides in Vietnam and East Asia, including China and the Korean peninsula. It is an important Chinese traditional herb to treat cough, asthma, tonsillitis, pharyngitis and tumor. In Vietnam, its rhizomes have been widely used as an anti-inflammatory, antitussive, and expectorant agent as well as against throat troubles.[8] Previous phytochemical studies on this plant led to the isolation of iridal-type triterpenoids, isoflavonoids, benzoquinones and benzofurans.[9]
In the present work, our further investigation on the EtOAc soluble fraction of the Belamcanda rhizome has resulted in the isolation of 11 compounds (1 – 11). Among those, compounds 2 – 4 have been isolated first time from Belamcanda Rhizome. Chemical structures of the isolated compounds were identified by analyzing UV-vis, [α]D, CD, IR, 1H and 13C-NMR, and mass spectroscopic data as well as by comparision of these spectroscopic data with reported in the literatures for 7 isoflavonoids named as iridin (1),[12] tectorigenin (5),[13] irilin D (6),[14] irisflorentin (8),[14] tectoridin (9),[13] iristectorin A (10),[15] iristectorigenin A (11),[16] an euflavonoid named as isorhamnetin-3-O-(6′′-acetyl-)-β-D-glucopyranosid1 (3),[17] (7R,8S)-dehydrodiconiferyl alcohol-9′γ-methyl ether (2), 1,3-O-diferuloylsucrose (4), and acetovanillone (7).[18]
MicroRNA (miRNA) are short endogenous non-coding RNAs (18-25 nucleotides length) that bind to the 3’UTR of certain mRNAs and involved in the regulation of mRNA stability and protein synthesis, and each miRNA can target multiple transcripts. Recent studies demonstrated the role of miRNA play a fundamental role in a number of human disease states, including inflammatory diseases. The biological significance of microRNA control of COX-2 expression in the macrophages Raw246.7 cells has not to be determined clearly. Recently, several report demonstrated that miR-146a, miR-146b, miR-155, miR-25 and miR-147 induced inflammatory activity.[19,20]
In this study, we initially investigated the effects of 4 fractions: hexan fraction, ethyl acetate fraction, buthanol fraction and methanol fraction of B. chinensis on COX-2 protein expression in Raw246.7 cells. Among them, ethyl acetate fraction showed has most affect to diminish COX-2 suppression. From ethyl acetate fraction, we isolated 11 compounds and tested the effect of these compounds on COX-2 expression and PGE2 production in RAW246.7 cells. Moreover, we observed the increasing of miR-146a, miR-146b, miR-155, miR-25 and miR-147 by LPS treatment in Raw246.7 cells. Finally, we also further demonstrated the cellular signaling pathways for the expression of LPS-induced COX-2 in response to BC-2 compound.
Materials and methods
Plant materials
The rhizomes B. chinensis were collected from Thanh Hoa province (Vietnam), in November 2011 and botanically identified by Dr. Pham Thi Thanh Huyen and Nguyen Quynh Nga, Department Herbarium, National Institute of Medicinal Materials (NIMM). A voucher specimen (VIMM2011–21) has been deposited at the Herbarium of NIMM, Hanoi, Vietnam.
Optical rotations were determined on a Rudolph Autopol IV polarimeter using a 100 mm glass microcell. UV spectra were recorded in MeOH on a JASCO V-550 UV/Vis spectrometer with a 0.5 nm resolution, and IR spectra (KBr) were obtained using a Nicolet 6700 FT-IR (Thermo Electron Corp.). NMR spectra were recorded on a Varian Unity Inova 500 MHz spectrometer with TMS as the internal standard. EIMS and HREIMS data were recorded on a Micromass QTOF2 (Micromass, Wythenshawe, UK) mass spectrometer. Silica gel (Merck, 63-200 mm particle size), RP-18 (Merck, 40–63 mm particle size), and Sephadex LH-20 were used for column chromatography. TLC was carried out with silica gel 60 F254 and RP-18 F254 plates.
Extraction and isolation
The dried rhizomes of B. chinensis (4.0 kg) were finely ground and extracted with hot MeOH (10 L × 2 times × 24 hrs) in a continuous extractor. The combined MeOH extracts were concentrated to yield a dry residue (298.0 g). This crude extract was then suspended in H2O (2 L) and partitioned successively with n-hexane (3 × 1.5 L), EtOAc (3 × 1.5 L), and n-BuOH (3 × 1.5 L). The EtOAc fraction (178.0 g), which exhibited strong anti-inflammatory activity, was chromatographed over a silica gel column (17 × 30 cm; 63–200 mm particle size) and eluted with gradient mixtures of CHCl3/MeOH (29:1, 28:2, 1:29, each 2.0 L) to yield eight pooled fractions (F1: 2.3 g; F2: 10.2 g; F3: 33.5 g; F4: 20.0 g; F5: 9.8 g; F6: 33.3 g; F7: 19.0 g and F8: 25.0 g). From fraction F2, the major compound iris A (11, 1.03 g) was crystallized out from n-hexane/EtOAc. The solution after crystallization of 11 was separated by an RP-18 column (7 × 50 cm; 40–63 mm particle size) and eluted with a stepwise gradient of MeOH/H2O (2:1 to 10:1) to afford seven subfractions (F2.1–F2.7). From fractions F2.2 (0.4 g) and F2.6 (3.2 g), compounds 7 (75.0 mg) and 8 (0.4 g) were crystallized out from MeOH respectively.
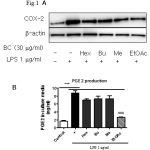 |
Figure 1: Comparison of COX-2 expression effects between four fractions (Hex: hexan, Bu: Buthanol, Me: methanol, EtOAc: Ethyl acetate) from B. chinensis. |
Eighteen hours after treating cells with LPS (1 µg/ml) with or without fraction in Raw264.7 cells. (A) Samples were harvested and lysated to immunoblottings with COX-2 and β-actin antibodies. Relative changes in the COX-2 expression were assessed by scanning densitometry (*significant as compared to control, *p < 0.05; #significant as compared to LPS group, n=3-5). (B) Comparison of PGE2 production effects between four fractions from B. chinensis. Raw264.7 cells were incubated with 1 μg/ml LPS for 24 h with or without fraction and amount of PGE2 in medium was determined using PGE2-specific ELISA assays (*significant as compared to control, *p < 0.05; #significant as compared to LPS group, n=3-5).
Fraction F4 was chromatographed over a silica gel column (7 × 30 cm) and eluted with gradient mixtures of n-hexan/EtOAc (5:1 to 5:5) to give five subfractions (F4.1–F4.5). F4.2 (0.8 g) was continuously purified by an RP-18 column (5 × 20 cm), with a stepwise gradient of MeOH-H2O (1:1 to 1:0) to afford compound 6 (195mg). Subfraction F4.3 (10.3 g) was chromatographed over a Sephadex LH-20 column (7 × 20 cm) using MeOH as the eluting solvent to give four subfractions (F4.3.1–F4.3.4). F4.3.3 (2.8 g) was futher purified by an RP-18 column (5 × 30 cm), with a stepwise gradient of MeOH-H2O (1:1 to 10:1) to yield compound 4 (15mg) and compound 5 (740 mg).
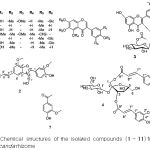 |
Figure 2: Chemical structures of the isolated compounds (1 – 11) from the Belamcanda rhizome |
Fraction F6 was chromatographed over a silica gel column (10 × 30 cm) and eluted with gradient mixtures of CHCl3/MeOH (10:1 to 0:1) to yield seven subfractions (F6.1–F6.7). F6.2 (3.2 g) was futher fractionated on an RP-18 column (7 × 40 cm), with a stepwise gradient of MeOH-H2O (1:1 to 10:1) to yield compound 3 (10.5 mg) and compound 10 (60 mg). Subfraction F6.3 (9.8 g) was purified by a Sephadex LH-20 column (10 × 30 cm) using MeOH as the eluting solvent to give compound 9 (5.2 g). Finally, compounds 1 (32 mg) and 2 (20 mg) were purified by an RP-18 column (5 × 40 cm), with a stepwise gradient of MeOH-H2O (1:2 to 1:0) from subfraction F6.5 (2.7 g).
 |
Figure 3: (A) Comparison of COX-2 expression effects between 11 compounds from B. chinensis. |
18 h after treating cells with LPS (1μg/ml) with or without compounds in Raw264.7 cells, samples were harvested and lysated to immunoblottings with COX-2 and β-actin antibodies (*significant as compared to control, *p < 0.05; #significant as compared to LPS group, n=3-5). (B) Comparison of PGE2 production effects between 11 compounds from B. chinensis. Raw264.7 cells were incubated with 1 μg/ml LPS for 24 h with or without compounds and amount of PGE2 in medium was determined using PGE2-specific ELISA assays (*significant as compared to control, *p < 0.05; #significant as compared to LPS group, n=3-5). (C) Cytotoxicity of BC-2 in Raw264.7 cells. Cells were seed in a 48-well plate and various concentrations of BC-2 were incubated for 24 h. Cell viability were estimated by MTT assay. (*significant as compared to control, *p < 0.05; #significant as compared to LPS group, n=3-5).
Materials
DMEM, trypsine, fetal bovine serum, were supplied by Gibco BRL (Grand Island, NY). COX-2 (1:1000, cat: 610204), and HSP90 (1:1000, cat: 610418) was obtained from BD Biosciences. Secondary mouse or rabbit HRP-conjugated antibodies (1:5000 or 1:10000, Cell Signaling, #7074, #7076). LPS (cat: L4391), dexamethasone (cat: D4902), meloxicam (cat: M3935) and MTT (cat: M2128) were purchased from sigma Aldrich. Control mimic and miR-146a-5p miScript miRNA Mimic were purchased from Qiagen.
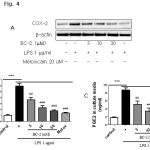 |
Figure 4: (A) Effect of BC-2 on COX-2 expression in dose-dependent manner (3-30 µM). |
Raw264.7 cells were treated with 1 μg/ml LPS for 24 h with or without BC-2 and then harvested and lysated to immunoblottings with COX-2 and β-actin antibodies. (B) Expression of COX-2 was analyzed by qPCR (*significant as compared to control, *p < 0.05; #significant as compared to LPS group, n=3-5). (C) Effect of BC-2 on PGE2 production. Raw264.7 cells were incubated with 1 μg/ml LPS for 24 h with or without BC-2 and amounts of PGE2 in medium was determined using PGE2-specific ELISA assays. (*significant as compared to control, *p < 0.05; #significant as compared to LPS group, n=3-5).
Cell culture
Raw 264.7 cells were obtained from the American Type Culture Collection (ATCC, Rockville, MD). Cells were cultured in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum (FBS), 100 units/ml penicillin, and 100 μg/ml streptomycin at 37°C in 5% CO2 – 95% air. For all experiments, cells were grown to 80%-90% confluency and subjected to no more than 20 cell passages.
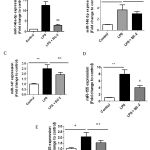 |
Figure 5: LPS increased miRNAs (miR-146a-5p, miR-146-3p, miR-147, miR-155 and miR-25). |
Raw264.7 cells were treated with 1 μg/ml LPS for 24 h with or without BC-2 (10 µM) and then harvested. miRNAs were extract and miRNAs expression was analyzed by qPCR (*significant as compared to control, *p < 0.05; #significant as compared to LPS group, n=3-5).
Western blot analysis
After treatment with plant extraction, fractions or compounds, cells were collected and washed with cold phosphate-buffered saline (PBS). The harvested cells were then lysed on ice for 30 min in 100 μl lysis buffer [120 mM NaCl, 40 mM Tris (pH 8), 0.1% NP40 (Nonidet P-40)] and centrifuged at 12,000 rpm for 30 min. Supernatants were collected from the lysates and protein concentrations were determined using the BCA protein assay kit (Pierce, Rockford, IL). Aliquots of the lysates (40 μg of protein) were boiled for 5 min and electrophoresed on 10% SDS-polyacrylamide gels. Proteins in the gels were transferred onto nitrocellulose membranes, which were then incubated with primary antibodies or mouse monoclonal β-actin antibodies. The membranes were further incubated with secondary anti-mouse or anti-rabbit antibodies. Finally, protein bands were detected using an enhanced chemiluminescence western blotting detection west femto kit (Thermo Scientific, Catalog #:34096).
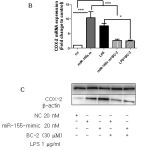 |
Figure 6: Role miR-146a and miR-155 on BC-2 suppressed LPS-induced COX-2 expression. |
Raw264.7 cells were transfected with 20 nM of negative control, miR-146a mimic or miR-155 mimic for 12 h and then stimulated with or without LPS and BC-2 compound for 18h. Cells were then harvested for qPCR and Western blot analysis (*significant as compared to control, *p < 0.05; #significant as compared to LPS group, n=3-5). (A-B) Role of miR-146a mimic (miR-146a-m), BC-2 compound with or without LPS on COX-2 protein and COX-2 mRNA expression. (C-D) Role of miR-155 mimic (miR-155-m), BC-2 compound with or without LPS on COX-2 protein and COX-2 mRNA expression.
Quantitative real-time PCR (qRT-PCR)
Total RNA was isolated using miRNeasy mini kit (Qiagen, #217004) according to the manufacturer’s instructions. The expression of miRNA and mRNA was analyzed by real-time qPCR (StepOnePlus qPCR cycler, Applied Biosystems) using miScript SYBR Green RT-PCR Kit Qiagen, (cat: 218073), miScript primer assays (Qiagen): Mm_miR-146a-5p (cat: MS00001638), Mm_miR_147 (cat: MS00032333), Mm_miR-155 (cat: MS00001701), Mm_miR-146a-3p (cat: MS00024220), Mm_miR-25 (cat: MS00001337) and QuantiFast SYBR Green RT-PCR Kit Qiagen, #204156) and QuantiTect primer assays (Qiagen): COX-2 (Ptgs2) (cat: QT00165347), Mm_GADPH (cat: QT01658692).
Enzyme-linked immunosorbent assay (ELISA)
Commercial ELISA kit (Cayman Chemical, Ann Arbor, MI) was used to determine prostaglandin E2 (PGE2) concentrations in culture medium according to the manufacturer’s protocols.
Statistics
Values are presented as mean ± S.E. unless otherwise stated. P-values were calculated by Student’s t-test or one-way analysis of variance followed by Bonferroni post-hoc testing using GraphPad Prism 5 (GraphPad Software Inc.). P<0.05 was considered statistically significant. Data are expressed as mean ± S.E.M. *, p<0.05; **, p<0.01; ***, p<0.001.
Results
Screening of COX-2 inhibitor from Belamcanda chinensis
COX-2 is increased in response to inflammatory stimuli and has been implicated in carcinogenesis of several neoplasms, development immunity, pain and inflammation.[1,2] In order to screen drug candidates exhibit anti-inflammatory activity, we tested the efficacy of each of four fractions (hexane fraction, methanol fraction, buthanol fraction and ethyl acetate fraction) from Belamcanda chinensis rhizomes at a 30 mg/mL on LPS-induced COX-2 protein expression in Raw246.7 cells. Among four fractions, ethyl acetate fraction most potently inhibited LPS-induced COX-2 protein expression and PGE2 production (Fig. 1A and 1B). From ethyl acetate fraction, eleven compounds were isolated (BC-1: Iridin-7-Glucopyranoside, BC-2: (7R,8S)-dehydrodiconiferyl alcohol-9′γ-methyl ether, BC-3: isorhamnetin-3-O-(6′′-acetyl-)-β-D-glucopyranosid1, BC-4: 1,3-O-diferuloylsucrose, BC-5: Tectorigenin, BC-6: Iridin, BC-7: Acetovanillone, BC-8: Irisflorentin, BC-9: Tectoridin, BC-10: Iristectorin A, BC-11: Iristectorigenin A) (Fig. 2) and we further examine the effects of eleven compounds (30 mM) and meloxicam (20 mM), dexamethasone (20 mM) on LPS-induced COX-2 protein expression and LPS-induced PGE2 production in Raw246.7 cells (Fig. 3). Western blot and real time-PCR assays revealed that 30 mM of compound BC-2 potently suppressed LPS-induced COX-2 expression in Raw246.7 cells (Fig. 3A and 3B). Furthermore, ELISA assay confirmed that BC-2 decreased the level of LPS-induced PGE2 production in Raw246.7 cells (Fig. 3B). We then assessed potential BC-2 compound cytotoxicity in Raw246.7 cells by MTT assay. Fig. 3C showed that BC-2 compound did not cause cytotoxicity in Raw246.7 cells at 30 mM. Thus, we used BC-2 in the 3-30 mM concentration range for subsequent experiments.
Effects of BC-2 compound on COX-2 expression and PGE2 production
To confirm the effects of BC-2 compound on LPS-induced COX-2 expression and PGE2 production, we performed western blot analysis, real time-PCR to detect changes in COX-2 expression and ELISA to measure PGE2 production in RAW246.7 cells in the presence of BC-2 (3-30 mM) or meloxicam (melox 20 μM). As expected, BC-2 compound inhibited LPS-induced COX-2 protein and mRNA expression in dose-dependent manner. In addition, we observed the effect of BC-2 at 30 mM on LPS-induced COX-2 expression as strong as meloxicam at 20 μM. This result is confirmed by measuring of PGE2 production. Both BC-2 at 30 mM and meloxicam at 20 μM completely suppressed PGE2 production level (Fig. 3). These data suggest the inhibition of COX-2 and PGE2 production by BC-2 compound.
Involvement of miR-146a and miR-155 in COX-2 repression by BC-2 compound
Recent studies showed that miRNAs are targets for drug discovery including anti-inflammatory theurapeutics.[19,20] To further elucidate the signaling pathways involved in BC-2 compound reduced LPS-induced COX-2 expression, we determined whether LPS with and without BC-2 compound affects miR-146a, miR-146b, miR-155, miR-25 and miR-147 expression by real time-qPCR. Fig. 5A-E presented the expression of miR-146a-5p, miR-146a-3p, miR-155, miR-25 and miR-147 were significantly increased by LPS treatment. However, only miR-146a-5p and miR-155 expression induced by LPS were abolished in the presence of BC-2 compound. To confirm this data, we tested the effect of miR-146a-5p mimic and miR-155 mimic on COX-2 protein and mRNA expression. As expected, miR-146a-5p mimic and miR-155 mimic significantly induced COX-2 protein and mRNA expression, and the COX-2 elevation by miR-146a-5p-mimic and and miR-155 mimic were diminished by BC-2 compound (Fig. 6). These results demonstrated the involvement of miR-146a-5p and miR-155 in the suppression of LPS-induced COX-2 in Raw246.7 cells by BC-2 compound.
Discussion
As part of an ongoing search for new anti-inflammatory agents from medicinal plants, we first demonstrated in this study the molecular pharmacology of compound BC-2 from Belamcanda chinensis against inflammatory activity through miR146a, miR-155/COX-2 in macrophages cells Raw246.7.
Rhizomes of Belamcanda chinensis have been widely used as a traditional medicinal plant for anti-inflammatory, antitussive, and expectorant agent as well as against throat troubles.[8] Numerous studies reported the chemical components of Belamcanda chinensis such as iridal-type triterpenoids, isoflavonoids, benzoquinones or benzofurans[8,9] and they have have diverse bioactivities, such as anti-cancer,[24] anti-inflammatory,[25] anti-oxidant and anti-diabetes effects.[27]
Previous report have shown that COX-2 is inhibited by tectorigenin, irigenin, tectorigenin and tectoridin.[25,28] Using eleven compounds from ethyl acetate fration of B. hinensis, we found for the first time that BC-2 potently suppressed LPS-induced COX-2 expression and PGE2 production in macrophages cells. We observed the effect of BC-2 compound on COX-2 expression and PGE2 production is potently strong as dexamethasone or meloxicam.
BC-2 compound was first found in cultures of Fusarium solani M-13-1 in the presence of substrate neolignan dehydrodiconiferyl alcohol (DHCA) in 1979 [31]. This is first report showed the pharmacological effect of BC-2 compound on anti-inflammatory activity.
miRNAs are short non-coding ribonucleic acids of about 20-24 nucleotides long whose existence in mammals. One miRNA has many targets and one target can be silenced by multiple different miRNAs gives them an enormous potential to regulate cellular phenotype at various levels. Recently, numerous demonstrated that miRNAs are new targets for of human disease states such as cancer, diabetes, liver or inflammatory diseases.[32,33] Here, we presented that LPS induced-miR-146a-5p, miR-146a-3p, miR-147, miR-155, miR-25 and BC-2 compound potently abrogated miR-146a-5p and miR-155 expression in response to LPS. Furthermore, miR-146a-5p and miR-155 mimic increased COX-2 expression in macrophages cells. However, these increasing were inhibited by BC-2 compound.
Conclusions
In summary, the present study showed that BC-2 compound is a potential suppressor of LPS-induced COX-2 expression and PGE2 production which in under the control of miR-146a-5p and miR-155. This finding has significant implications for the elucidation of the pharmacological mechanisms underlying the beneficial effects of BC-2 compound in Raw 264.7 macrophages and BC-2 compound could become a therapeutic agent for the treatment or prevention of inflammation disease. Guardiola-Serrano F, Beteta-Göbel R, Rodríguez-Lorca R, Ibarguren M, López DJ, Terés S, Alvarez R, Alonso-Sande M, Busquets X, Escribá PV. J. Pharmacol. Exp. Ther. 2015 354: 213-224.
References
- Goetzl EJ, An S, Smith WL. FASEB. J. 1995 9: 1051–1058.
- Arias-Negrete S et al. Proinflammatory cytokines regulate cyclooxygenase-2 mRNA expression in human macrophages. Biochem Biophys Res Commun 1995; 208: 582–589.
- Tsuji S, Tsujii M, Kawano S, Hori M. J. Exp. Clin. Cancer Res. 2001 20: 117–129.
- Thuong PT, Dao TT, Pham TH, Nguyen PH, Le TV, Lee KY, Oh WK. J. Nat. Prod. 2009.; 72: 2040-2042.
- Choi RJ, Chun J, Khan S, Kim YS. Int. Immunopharmacol 2014 18: 182-190.
- Monthakantirat O, De-Eknamkul W, Umehara K, Yoshinaga Y, Miyase T, Warashina T, Noguchi H. J. Nat. Prod. 2005 68: 361-364.
- Fukuyama Y, Okino J, Kodama M. Chem. Pharm. Bull (Tokyo) 1991 39: 1877-1879.
- Ali AA, El-Emary NA, El-Moghazi MA, Darwish FM, AW Frahm. Phytochemistry 1983 22: 2061- 2063.
- Kim C, Shin S, Ha H, Kim JM. Arch. Pharm. Res. 2003 26: 210-213.
- Liu M, Yang S, Jin L, Hu D, Wu Z, Yang S. Molecules 2012 17: 6156-6169.
- Sun Y, Li W, Wang J. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011 879: 975-980.
- Roger B, Jeannot V, Fernandez X, Cerantola S, Chahboun J. Phytochem. Anal. 2012 23: 450-455.
- Merfort I, Wendisch D. Planta Med. 1987 53: 434-437.
- Tan RX, Jakupovic J, Jia ZJ. Planta Medica. 1990; 56: 475 -477.
- Cheng Y, Kuang W, Hao Y, Zhang D, Lei M, Du L, Jiao H, Zhang X, Wang F. Inflammation 2012 35: 1308-1313.
- Cheung ST, So EY, Chang D, Ming-Lum A, Mui AL. PLoS One 2013; 8: e71336.
- Thelen P, Scharf JG, Burfeind P, Hemmerlein B, Wuttke W, Spengler B, Christoffel V, Ringert RH, Seidlová-Wuttke D. Carcinogenesis 2005 26: 1360-1367.
- Kim YP, Yamada M, Lim SS, Lee SH, Ryu N, Shin KH, Ohuchi K. Biochim. Biophys. Acta. 1999 1438: 399-407.
- Wu C, Li Y, Chen Y, Lao X, Sheng L, Dai R, Meng W, Deng Y. Phytomedicine 2011 18: 292-297.
- Ahn KS, Noh EJ, Cha KH, Kim YS, Lim SS, Shin KH, Jung SH. Life Sci. 2006 78: 2336-2342.
- Ohta M, Higuchi T, Iwahara S. Arch. Microbiol. 1979 121: 23-28.
- McManus MT. Semin Cancer Biol. 2003 13: 253-258.
- Plaisance V, Waeber G, Regazzi R, Abderrahmani A. J. Diabetes Res. 2014 2014: 618652.







