Manuscript accepted on :December 10, 2016
Published online on: --
Plagiarism Check: Yes
Fawiziah Khalaf Alharbi and Zeinab Zakaria Saleh
Department of Biology Science, College of Science and Arts, Qassim University, Saudi Arabia.
Corresponding Author E-mail: foz381@hotmail.com
DOI : https://dx.doi.org/10.13005/bpj/1038
Abstract
This study was conducted to investigate the ultrastructural postnatal changes occurring inthe media of four different arteries. Twenty-five albino rats of both sexes of five different ages (oneday, one week, one month, adult & senile) were used for this study. From each rat, four arteries weredissected, basilar, renal, uterine and femoral for electron microscopic study.In one-day old rat arteriesthe media was formed of only one layer of smooth muscle cells which increased gradually in thickness with the advanced agetill reaching five to six layers in the adult rat arteries except in adultrat femoral which was formed of nearly 6-7 layers with apparent crowding of the cells. The media ofone day old arteries was formed of smooth muscle cells rich in cytoplasmic organelles mainlymitochondria,rough endoplasmic reticulum and poorly developed myofilaments. As age advanced,the cell organelles decreased in amount while the myofilaments increased in number and becamemore developed till the adult age in which the cell organelles were limited to only few perinuclearmitochondrial aggregates but with large amount of well- developed myofilaments. In the senile agethe smooth muscles became paler in color due to a decrease in the density of the myofilaments.Vacuoles started to appear of studied arteries at different ages but in all arteries, vacuoles increasedwith the advance of age and especially in senile age. External elastic lamina was apparent as early as the age of one day in both renal and femoral arteries, while it was apparent in the age of one weekin uterine artery. In the basilar artery, there was only basal lamina instead of external elasticlamina.
Keywords
perinuclearmitochondrial; myofilaments
Download this article as:| Copy the following to cite this article: Alharbi F. K, Saleh Z. Z. A Comparative Study of Tunica Media of Some Arteries in Postnatal Rats. Biomed Pharmacol J 2016;9(3). |
| Copy the following to cite this URL: Alharbi F. K, Saleh Z. Z. A Comparative Study of Tunica Media of Some Arteries in Postnatal Rats. Biomed Pharmacol J 2016;9(3). Available from: http://biomedpharmajournal.org/?p=11061 |
Introduction
A lot of experimental work was done on arteries to examine the phenomenon of atherosclerosis and its predisposing factors. These studies showed that atherosclerosis was a disease beginning in infancy and progressing rapidly during the first and second decades of life and becoming symptomatic in the adult. It was also accompanied with intimal proliferation and thickening together with modifications of typical smooth muscles into a typical ones (González etal, 2001; Lee,2005; Shaun etal,2009 ; Hongyu etal,2010;Yanhua, and Qingbo, 2011).
Many workers studied the structure of the adult media with its smooth muscle cells. They came to the conclusion that the smooth muscle cells were arranged in a helical fashion around the vessel and that the myofilaments formed characteristic cytoplasmic components of the vascular smooth muscle together with the presence of perinuclear mitochondria aggregates (Juan etal,2009;Bode etal,2012; and Sridevi etal, 2013). In the renalartery, the elastic lamellae of the media became thinner as the vessel size decreased and they disappeared almost completely distal to the second branch point. In addition, collagen fibrils were present in the extracellular space between smooth muscle cells, but became less densely packed as the vessel decreased in size. The predominant orientation of these collagen fibrils appeared to parallel to the long axis of the renal artery although fibrils were observed in all directions (Fatemeh, 2012, and Lwakiri et al, 2014).
Studies showed that the femoral artery was of muscle- elastic type with discontinuous elastic laminae in tunica media. Overall tridimensional architecture of the elastic tissue in the femoral artery had been studied by scanning electron microscopy. It proved to be composed of two distinct inner and outer sheet – like laminae bridged by a dense continuous interlaminar network of elastic fibers. Tunnel – like compartments free of elastic tissue extended helically into the medial wall of the of the femoral artery (Layne et al, 2012). The smooth muscle cell in the media of femoral artery often had spindle- shaped overall appearance with the outlines more or lessirregular. The nucleus was found to be in the widest part of the cell (Todd et al, 2011).
There were three major aspects of the effect of aging upon vascular smooth muscle. The appearance of degenerative changes within smooth muscle itself, the alterations in the connective tissue components that were normally produced by vascular smooth muscle, and the migratory and proliferation changes of smooth muscle within the intima leading to intimal thickening (Yanhua, and Qingbo, 2011). The impairment of morphogenetic function of vascular smooth muscle by aging was manifested by an increase of connective tissue elements particularly collagen, relative to smooth muscle (Pasqualino andBourne, 2016). The functional result of these alteration was the decrease in vascular elasticity with age (Pijnenborg etal, 2006,and Obimbo etal, 2012).
Electron microscope of spontaneous and induced lesions had proved the involvement of smooth muscle in atherosclerosis (Shaun etal, 2009). Modifications of typical smooth cells into a typical ones involved progressive dilatation of the endoplasmic reticulum and an accompanying increase of ribosomes, vacuolation and increase in the number of mitochondria and crowding of myofilaments towards the periphery of the cell. Lipid droplets appeared within the smooth muscle cells before the appearance of extracellular lipids. The increasing lipid infiltration with progressive loss of myofilaments might transform the smooth muscle into foam cells (Juan etal,2009, and Hongyu etal, 2010). The structure of arteries had been subjected to many studies either by light or by electron microscope. Reviewing the literature, it was evident that most of the studies had been done on the adult arteries. Besides, the histological studies of development of the arteries were few in the literature. The aim of the present work was done to study the structural changes during postnatal development in the four different arteries from the age of one day till the senile age using electron microscope. The four chosen arteries were, the basilar artery which is an artery of one of the most vital organ, the renal artery, a high blood flow artery, the uterine, a hormonal dependent and tortuous artery, and the femoral artery one of the peripheral arteries.
Materialand Methods
Twenty – five albino rats were used. The rats were of five age groups (one day, one week, one month, adult and senile), five rats for each group. From each rat, four arteries were dissected; basilar renal, uterine and femoral. The selected arteries were rapidly put 3% gluteraldehyde for two hours, then, the specimens were further processed for electron microscopy according to (Bancroft and Gamble,2008).
The ultrathin sections were cut and picked up on copper grids. The sections on the grids were double stained using uranyl acetate and load citrate stains according to (Hayat, 2000).
Results
In one-day old rat basilar artery, the media was formed of a single layer of circularly disposed smooth muscle cell. The smooth muscle cell contained small irregular nucleus with peripheral chromatin condensation and fewmyofilaments in the form of actin and myosin. The smooth muscle cells were widely separated by irregular intercellular spaces which contained dispersed collagen fibers. The media was separated from the intima by non-homogenous internal elastic lamina and from the adventitia by basal lamina (Fig.1). In one-day old rat renal artery, the media was formed of one cell layer of smooth muscle cell which had large irregular nuclei and were rich in cytoplasmic organelles especially mitochondria and ribosomes with small bundles of myofilaments. The media was separated from intima by patchy foamy internal elastic lamina and from the adventitia by an incomplete external elastic lamina (Fig.2). In one-day old rat uterine artery, the media was formed of one cell layer of smooth muscle cells which contained two nuclei and were rich in cytoplasmic organelles especially mitochondria glycogen granules and free ribosomes. The media was separated from intima by irregular non – homogenous internal elastic lamina and from adventitia by ill-developed external elastic lamina (Fig.3). In one-day old rat femoral artery, the media was formed of a single layer of smooth muscle cells, which contained large irregular nuclei with peripheral chromatin condensation and dense cytoplasm containing few myofilaments in the form of actin filaments and patches of myosin. The cells contained a plenty of cytoplasmic organelles especially mitochondria and free ribosomes. The nucleiwere separated from intimaby non – homogenous internal elastic laminaand from the adventitia by under-developed external elastic lamina (Fig. 4).
 |
Figure 1: Electromicrograph of one-day old rat basilar artery showing the media formed of one layer of smooth muscle cells widely separated by irregular intercellular space (arrow) containing collagen fibers (C). The smooth muscle cells contained nucleus (N) and few myofilaments in the form of actin (Ac) and myosin (M). Notice internal elastic lamina (IL) and basal lamina (B). (X10000) |
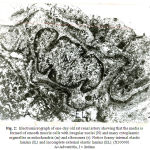 |
Figure 2: Electromicrograph of one-day old rat renal artery showing that the media is formed of smooth muscle cells with irregular nuclei (N) and many cytoplasmic organelles as mitochondria (m) and ribosomes (r). Notice foamy internal elastic lamina (IL) and incomplete external elastic lamina (EL). (X10000) A=Adventitia, I = Intima |
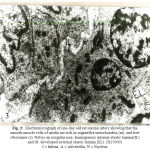 |
Figure 3: Electromicrograph of one-day old rat uterine artery showing that the smooth muscle cells of media are rich in organelles mitochondria (m), and free ribosomes (r). Notice an irregular non- homogenous internal elastic lamina(IL) and ill- developed external elastic lamina (EL). (X15000) I = Intima, A = Adventitia, N = Nucleus |
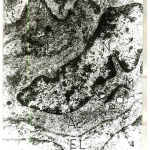 |
Figure 4: Electromicrograph of one-day old rat femoral artery showing smooth muscle cell of media with a large irregular nucleus (N) with peripheral chromatin condensation and dense cytoplasm containing bundle of actin filaments (Ac) together with few patches of myosin (M), mitochondria (m) and free ribosomes (r). Notice non-homogenous internal elastic lamina (IL) and under –developed external elastic lamina (EL). (X15000) |
In one-week old rat basilar artery, the media was formed of few layersof smooth muscle cells with irregular cell membranes and wide intercellular spaces. The smooth muscle cells contained irregular nuclei, mitochondria and plenty actin filaments interposing with small patches of myosin. The internal elastic lamina became more homogenous and the basal lamina became well – developed. (Fig.5). In one-week old rat renal artery, the media showed an interrupted intermediate elastic lamina with increased density of both internal and external elasticlaminas. The muscle cells were rich in myofilaments in the form of plenty of actin with few patches of myosin (Fig.6). In one-week old rat uterine artery, the media was formed of smooth muscle cells which showed increased myofilament deposition and perinuclear mitochondrial aggregates (Fig.7), together with the presence of an interrupted elastic lamina between the two layers of smooth muscles cells (Fig.8). There were increased density of both external and internal elastic lamina (Figs. 7×8). In one-week old rat femoral artery, the media was packed with well-developed smooth muscle cells which surrounded by minimal elastic tissue. The media showed increased density of the smooth muscle cells which were enclosed between corrugation of internal elastic lamina together with the presence of vacuoles in some of the muscle cells (Fig.9).
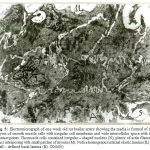 |
Figure 5: Electromicrograph of one-week old rat basilar artery showing the media is formed of few layers of smooth muscle cells with irregular cell membrane and wide intercellular space with few contact points.The muscle cells contained irregular – shaped nucleus (N), plenty of actin filaments (Ac) interposing with small patches of myosin (M).Notice homogenous internal elastic lamina (IL) and well – defined basal lamina (B). (X4000) |
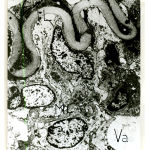 |
Figure 6: Electromicrograph of one-week old rat renal artery showing that the smooth muscle cells of the media contained actin filaments (Ac) and few patches of myosin (M). Notice the increased density of both internal elastic lamina (IL) and external elastic lamina (EL) and appearance of an incomplete intermediate elastic lamina (arrows). Notice also presence of vacuoles (Va). (X4000) |
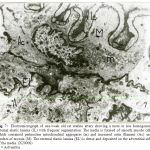 |
Figure 7: Electromicrograph of one-week old rat uterine artery showing a more or less homogenous internal elastic lamina (IL) with frequent segmentation. The media is formed of smooth muscle cells which contained perinuclear mitochondrial aggregates (m) and increased actin filament (Ac) and patches of myosin (M).The external elastic lamina (EL) is dense and deposited on the adventitial side of the media. (X20000) A = Adventitia |
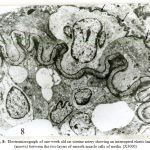 |
Figure 8: Electromicrograph of one-week old rat uterine artery showing an interrupted elastic lamina (arrows) between the two layers of smooth muscle cells of media. (X3000) |
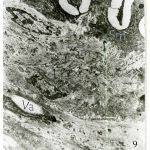 |
Figure 9: Electromicrograph of one-week old rat femoral artery showing a well- developed corrugated internal elastic lamina (IL) with points of interruptions.The media is packed with well- developed smooth muscle cells (Sm) and surrounded by minimal elastic tissue (arrows). Notice the increased density of smooth muscle cells (Sm`) enclosed between corrugation of internal elastic lamina. Notice also the presence of vacuoles (Va) in some cells. (X5000) |
In one-month old rat basilar artery, the media was formed of smooth muscle cells which became tightly packed with interdigitating between the adjacent cells so that cellular outlines became corrugated. The muscle cells were rich in perinuclear mitochondrial aggregates with myofilaments in the form of dense myosin filaments amongst plenty of actin filaments (Fig.10). In one-month old rat renal artery the media showed well-developed homogenous internal, middle and external elastic lamina, together with dispersed patches of elastic tissue among muscle cells of the media. Four – to – five cell layers of smooth muscle cells can be recognized (Fig.11). In one-month old rat uterine artery, the media was formed of four – to – five cell layers of pale and dark smooth muscle cells separated by wide intercellular spaces containing irregular bounds of elastic tissues.A well – developed corrugated internal and external elastic lamina can be recognized (Fig.12). In one-month old rat femoral artery, the media was packed with five – to – seven cell layers of vertically disposed smooth muscle cells with minimal elastic tissue in between. There were dark smooth muscle cells close to the internal elastic lamina and light smooth muscle cells away from it (Fig.13). The cells had large irregularnuclei with peripheral chromatin condensation, perinuclear mitochondrial aggregates and plenty of actin and myosin myofilaments (Fig.14).
 |
Figure 10: Electromicrograph of one month old rat basilar artery showing smooth muscle cells (Sm) of media containing perinuclear mitochondrial aggregates (m) together with dense myosin filament (M) amongst plenty of actin filament (Ac) . Notice the corrugated intercellular outlines. (X7000) |
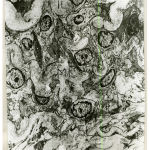 |
Figure 11: Electromicrograph of one-month old rat renal artery showing deposition of elastic tissues in internal (IL), intermediate (ML) and external (EL) elastic lamina. (X3000) |
 |
Figure 12: Electromicrograph of one-month old rat uterine artery showing that the media is formed of four-to-five cell layers of pale (Sm) and dark (Sm`) smooth muscle cells separated by irregular bounds of elastic tissue (arrows). Notice well-developed internal (IL) and external (EL) elastic lamina. (X3000) |
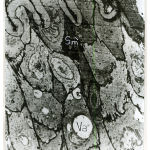 |
Figure 13: Electromicrograph of one-month old rat femoral artery showing that the media is packed with vertically disposed smooth muscle cells with minimal elastic tissue in between. Notice presence of dark smooth muscle cells (Sm`) close to internal elastic lamina (IL) and light smooth muscle cell (Sm) away from it. Notice presence of intracellular vacuole (Va). (X5000) |
In the adult rat basilar artery, the smooth muscle cells of the media became more dense with less evident internal structures. Multiple large vacuoles appeared in the smooth muscles of the media particularly the basal ones. No basal lamina was apparent between media and adventitia (Fig.15). In the adult rat renal artery, the media contained a well – developed internal, middle and external elastic lamina. The media contained smooth muscle cells, some of them were pale and others had dense myofilament in the form of actin and myosin with perinuclear mitochondrial aggregates (Fig.16). In the adult rat uterine artery, the media was formed of circularly disposed smooth muscle cells surrounded with patchy irregular deposition of elastic tissue. The smooth muscle cells were rich myofilments in the form of plenty of actin and myosin (Fig.17). In the adult rat femoral artery, the smooth muscle cells of the media surrounded by patches of elastic tissue and contained perinuclear mitochondrial aggregates together with plenty myofilaments in the form of plenty of actin and myosin filaments. A well-developed external elastic lamina limited the outer surface of the media from adventitia (Fig.18).
 |
Figure 14: Electromicrograph of one-month old rat femoral artery showing smooth muscle cells of media containing irregular nuclei (N) with peripheral condensation,perinuclear mitochondrial aggregates (m), Notice the presence of patches of elastic tissues between the cells (arrows) and intracellular filament of actin (Ac) and patches of myosin (M). (X10000) |
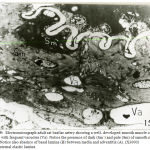 |
Figure 15: Electromicrograph adult rat basilar artery showing awell- developed smooth muscle cells of media with frequent vacuoles (Va). Notice the presence of dark (Sm`) and pale (Sm) of smooth muscle cells.Notice also absence of basal lamina (B) between media and adventitia (A). (X3000) IL = internal elastic lamina |
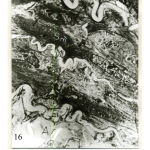 |
Figure 16: Electromicrograph adult renal artery showing well- developed homogenous internal (IL), middle (ML) and external (EL) elastic lamina together with dispersed patches of elastic tissue between smooth muscle cells of the media. Notice presence of dark (Sm`) and pale (Sm) smooth muscle cells. (X3000) |
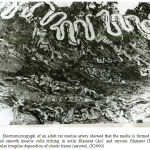 |
Figure 17: Electromicrograph of an adult rat uterine artery showed that the media is formed of well-developed smooth muscle cells riching in actin filament (Ac) and myosin filament (M), with intercellular irregular deposition of elastic tissue (arrows).(X3000) |
In the senile rat basilar artery, the media was formed of dense smooth muscle cells,others became less electron dense and some showed vacuolation (Fig. 19). In the senile rat renal artery, the media was formed of inner dense smooth muscle cells and outer rarified and vacuolated ones. The external elastic lamina becameirregularinterrupted andrarified (Fig.20). In the senile rat uterine artery, the media was formed of smooth muscle cells in which some cells were dark without apparent details, other cells were pale and contained rarified myofilaments and athird group of cells with vacuoles occupying most of the cytoplasm. A corrugated thin external elastic lamina separated the media from the adventitia (Fig.21). In the senile rat femoral artery, the media showed increased density of the smooth muscle cells close to the internal elastic lamina together with vacuolation of its cytoplasm. Minimal elastic tissue inbetween the smooth muscle cells produced the side corrugations of the smooth muscle cells (Fig.22).
 |
Figure 18: Electromicrograph of adult rat femoral artery showing smooth muscle cells of media surrounded by patches of elastic tissue (arrows) and containing perinuclear mitochondrial aggregates (m) together with filaments of actin (Ac) and myosin(M) and vacuoles (V) . Notice presence of well-developed external elastic lamina (EL).(X7000) |
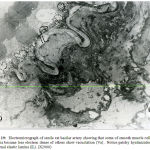 |
Figure 19: Electromicrograph of senile rat basilar artery showing that some of smooth muscle cells of media became less electron dense of others show vacuolation (Va) . Notice patchy hyalinization of internal elastic lamina (IL). (X2000) |
 |
Figure 20: Electromicrograph of senile rat renal artery showing the media containing inner dense smooth muscle cells (Sm`) and outer rarified and vacuolated cells (Sm). Notice thin and rarified external elastic lamina (EL). (X10000) |
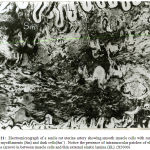 |
Figure 21: Electromicrograph of asenile rat uterine artery showing smooth muscle cellswith rarified pale myofilaments (Sm) and dark cells(Sm`) . Notice the presence of intramuscular patches of elastic tissue (arrow)in between muscle cells and thin external elastic lamina (EL). (X5000) |
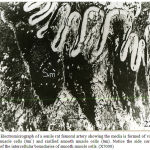 |
Figure 22: Electromicrograph of a senile rat femoral artery showing the media is formed of vacuolated smooth muscle cells (Sm`) and rarified smooth muscle cells (Sm). Notice the side corrugations (arrows) of the intercellular boundaries of smooth muscle cells. (X7000)
|
Discussion
In the present study, the media of each one of the one-day old rat arteries namely, the basilar, renal, uterine and femoral, was formed of one cell layer of smooth muscle cells surround by minimal elastic tissue. With the advance of age, the layers of the media increased in varying from four- to five cell layers in basilar, renal and uterine arteries and reaching six-to eight cell layers in femoral artery. The smooth muscle cells were arranged mostly in a circular fashion with inner vertically arranged cells in some arteries. In the femoral artery, most of cells were vertically arranged due to overcrowding of muscle cells. In agreement with the results of the present study, Sreenivasulu etal (2011) reported that the media of the most arteries composed of circularly arranged smooth muscle cells.Angel(2012) stated that the muscle cells of the most arteries were arranged in a helical fashion around the vessel.
In the present study, the smooth muscle cells of the media of one day old specimens were rich in cytoplasmic organelles in the form of fragments mitochondria, rough endoplasmic reticulum and free ribosomes. Such organelles were much more frequent andprominent than in the adult arteries. On the other hand, the myofilaments were poorly developed if compared with adult aged arteries. With the advance of age, the myofilaments in the smooth muscle cells increased gradually in amount and maturation together with a decrease in cytoplasmic organelles to few perinuclear mitochondrial aggregates in adult age. This was in agreement with findings ofHongyu etal (2010) in aorta of young mice and growing rats andPasqualino etal (2016) in aorta of infants and young children. The authors detected a transition between smooth muscle cells with many cytoplasmic organelles but scanty myofilaments and with advance of age the cytoplasmic organelles decreased in amount and the myofilaments became well developed.
In the present work, vacuoles started to appear in the smooth muscle cells of the studied arteries at different ages, which varied from one week as in renal and femoral arteries to one month as in femoral artery, but in all arteries, the vacuoles increased with advance of age and especially in senile age. These vacuoles showed also difference in their sizes and shape. In agreement with the results of the present work,Layne etal(2012) reported that the vacuoles were observed by light and the electron microscope in the smooth muscle cells of the media of the normal uterine and spermatic arteries of rat. By the light microscope, the vacuoles appeared as intracellular inclusions that were about the size of a nucleus, rounded or oval. Most the vacuoles were clear. By the electron microscope, they could be identified as myelin figures. Other vacuoleswere filled with a homogenous material which is stained more lightly than the cell itself. The authors added that, the pathogenesis of the vacuoles could be explained as a hernia from the cells into their neighbors.
In senile age, some smooth muscle cells became pale in color than others and this might be due to a decrease in the density and rarefaction of myofilaments in some smooth muscle cells if compared with others. In agreement with the results of the present study,Philip etal (2012)found that, in the media of thoracic and abdominal aortae of a fifty-eight years old human specimen, the smooth muscle cells of the media became round due to partial fragmentation of the cytoplasm. The myofilaments became loose in the cytoplasm of the smooth muscle cells. The thoracic aortic media from a sixty-eight years old human specimen revealed considerable degeneration and an irregular shape of the smooth muscle cells.
In the studied arteries, renal, uterine and femoral arteries showed deposition of elastic tissue in between the smooth muscle cells. In the renal artery, the deposition of elastic tissue appeared since the age of one month in the form of an intermediate elastic lamina dividing the media into two portions. The change continued till senile age. In agreement with the results of present study,Fatemeh (2012) stated that the renal artery in mammals showed a gradual transition from an elastic type at its origin with the presence of several elastic lamellae in the media to a muscular type after to second branching at the hilus.
In the present study, elastic tissue deposition started to appear from the age of one week in the form of irregular patches in between the smooth cells, which continued till senile age. In agreement with the present study,Obimbo etal (2012) reported that the arteries of the uterus were one of the muscular arteries andthat the intercellular substance holding the smooth muscle cells together in these arterieswas made by the smooth muscle cells themselves and it was chiefly made of elastin. In the present study, the elastic tissue of the femoral artery started to appear in between smooth muscle cells since the age of one week. This elastic tissue increased, then it became squeezed in between the overcrowded smooth muscle cells. This was in agreement withLayne etal (2012) who stated that the femoral artery was of the muscle- elastic type with discontinuous elastic laminae in the tunica media.
In the present work, the basilar arteries, starting from one day and up to the senile age, lacked the external elastic lamina and only the basal lamina was present between the media and adventitia. In adult and senile ages, the basal lamina was absent. This might be explained byQi etal (2014) was stated that within the cranial cavity where the vessels were protected from external pressure and tension, the cerebral arteries had relatively thin walls. The internal elastic lamina was well- developed but the media was thin and devoid of any elastic fibers. In the present work, in renal and femoral arteries the external elastic lamina was apparent as early as the age of one day and continued till the senile age. While in the uterine artery, external elastic lamina started to appear at the age of one week. At first, this lamina was poorly developed in the form of thin elastic lamina with some interruptions. It became thick and well- developed in adult rats. In the senile rats, the external elastic lamina became thin and irregular. In agreement with the results of the present study,Yanhua and Qingbo (2011) reported that the poor development of external elastic lamina at early ages might be due to poor development of elastin at this early ages, which gradually increased in amount with advance of age. In senile age, there was alteration in molecular conformation of elastin, which occurred due to aging process.
References
- Angel, Vod. (2012): Structure and function of smooth muscle with special reference to mast cells.J. Veterin.(9): 397- 443.
- Bancroft, J. D .and Gamble, M. (2008): Theory and Practice of Histological Techniques., Churchill Livingstone;6th edition.
- Bode,S. J.; Schmidt , A.; Gunther, D.; Stuhrmann, M. and Fieguth, A. (2012 ) : Aortic dissecting aneurysms-Histopathological findings. Foren. Scien. J. Vol. 214. P. 13-17.
- Fatemeh, R. N. (2012) : A microscopic and stereological study of the renal artery transitional zone of the adult male dog. Tur.J. of Veterin. and Anim. Scien. 36(2).
- González, MC. ; Arribas, SM. ; Molero, F. and Fernández, A. MS. (2001): Effect of removal of adventitia on vascular smooth muscle contraction and relaxation. Am. J. Physiol. Heart Circ. Physiol. 280(6): 2876- 81.
- Hayat, MA. (2000): Principles and techniques of electron microscopy: biological applications. 4th Ed. pp. 543. Cambridge, U.K.; New York: Cambridge University Press, 2000.
- Hongyu,Qiu. ; Ya, Zhu. ; Zhe, Sun. and Jerome, P. Trz. (2010): Vascular smooth muscle cells stiffness in aorta with aging. Circulation Research (107): 615- 619.
- Juan, P.; Hernández,F. ; Jaimar, R.; Adriana, P.; Ninoska, V.; José, L.; Arcaya, E. C. and Jesús, M. (2009) : Structural and Ultrastructural Analysis of Cerebral Cortex, Cerebellum, and Hypothalamus from Diabetic Rats. J. of Cerebral Blood Flow.
- Layne, AJ.; Fairman, RM.; Jackson, BM. ; Woo, EY.; Davis, JT.; Mohler, ER. And Wang, GJ. (2012): Analysis of femoral artery intima-media thickness during the cardiac cycle. J. Surg. Res., 177(2): 382-6.
- Lee, RM. (2005): Morphology of cerebral arteries. Pharmacol. Ther., 66 (1): 149 – 73.
- Lwakiri,T.; Sato,Y.; Matsuura,Y.; Hatakeyama,K. ; Marutsuka,K.; Yamashita,A.; Fujimoto,S, Kitamura,K and Asada,Y. (2014): Association between renal vasculature changes and generalized atherosclerosis. J. Atheroscler. Thromb., 21(2):99-107.
- Obimbo, MM. ; Ogeng’o, JA. And Saidi, H.(2012): Comparative regional morphometric changes in human uterine artery before and during pregnancy. Pan. Afr. Med. J. (3).
- Pasqualino, A. and Bourne, G.H.(2016) : Histochemical studies of fetal arteries of Koreans with special reference to atherogenesis in adults. J. Neuro. Surg. 4(1): 39- 42.
- Philip, I. A.; Jeremy, P. T. W. and Michelle, J. C. (2012) : ” The Cardiovascular System at a Glance “. Vascular histologyand smooth muscle cell ultrastructure 4thEdition, Wiley – Blackwell
- Pijnenborg, R. ; . Vercruysse, L.and Vercruysse, L. (2006) :The uterine spiral arteries in human pregnancy: facts and controversies. J. Obset. & Gyna. 10(27) : 939-958.
- Qi,Q.; Liu,X.; Zhang,G.; He,W.; Ma,R.; Cong,B. and Li,Y. (2014): Morphological changes of cerebral vessels and expression patterns of MMP-2 and MMP-9 on cerebrovascular wall of alcoholic rats. Int.J.Clin. Path., 7(5):1880-8.
- Shaun, L.S. ; Danusia, J. G. and Robert, M. K. (2009) : Arterial internal elastic lamina. J. Anat. 214(4): 258 – 266.
- Sreenivasulu,R.;Pramod, K. and Keerthana, P. (2011): Histomorphometric and sympathetic innervation of the human internal thoracic artery. J. Clin. 66(1).
- Sridevi, N.; Adam, K.; Cam, H. Donald, G. and Nikolaos, M. (2013): Role of micropjections in my endothelial feedback – a theoretical study. J. Physio. 11(591) :2795- 2812.
- Todd, M. E.; Laye C. G. and Osborne, D. N. (2011): The dimensional characteristics of smooth in rat blood vessels circ. Res. 116: 53- 64.
- Yanhua, Hu. and Qingbo, Xu. (2011):Arteriosclerosis, Thrombosis, and Vascular Biology.(31): 1523- 1529.







