Manuscript accepted on :June 25, 2016
Published online on: 12-08-2016
Plagiarism Check: Yes
Tran Thi Hien1,5*, Do Thi Ha3, Do Minh Truong2, Loi Vu Duc4, Trong Tuan Dao3 and Nguyen Thanh Hai4
1Department of pharmacy, Thai Binh medical and pharmacy University, Vietnam.
2College of Sciences, Thai Nguyen University, Vietnam.
3National Institute of Medicinal Materials, 3B Quangtrung, Hanoi, Vietnam.
4School of Medicine and Pharmacy, Hanoi National University, 144 Xuan Thuy street, Cau Giay, Hanoi, Vietnam.
5Faculty of medicine, Lund University, Sweden.
*Corresponding Author E-mail: nguyenthanh.haihnu@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/967
Abstract
p53, a tumor suppressor protein, has important roles in DNA repair, cell cycle and apoptosis, is a one of the key events in cancer development. Coix lacryma-jobi seed has been used as a food and traditional medicine plant with anti-oxidant, anti-cancer and anti-diabetic effects. In currently research, we identified the most potent p53-increasing compound among 4 compounds (1 – 4) found in Coix lacryma-jobi and demonstrated its molecular mechanism in MCF-7 cells. Among the four isolated compounds (1 – 4), triolein most increased p53. Triolein treatment induced p53, p21, p27 and Bax in MCF-7 cells. Moreover, triolein caused S phase arrest through suppression of CDK1, phopho-Rb and E2F1 in dose-dependent manner. We also observed the decreasing of DNA synthesis by triolein. These data suggest that triolein may induced cell cycle restart involve DNA synthesis and apoptosis pathway in MCF-7 cells.
Keywords
Triolein; Coix lacryma-jobi; MCF-7 cells; apoptosis; DNA synthesis
Download this article as:| Copy the following to cite this article: Hien T. T, Ha D. T, Truong D. M, Duc L. V, Dao T. T, Hai N. T. Triolein from Coix Lacryma-Jobi Induces Cell Cycle Arrest through P53/P21 Signaling Pathway. Biomed Pharmacol J 2016;9(2). |
| Copy the following to cite this URL: Hien T. T, Ha D. T, Truong D. M, Duc L. V, Dao T. T, Hai N. T. Triolein from Coix Lacryma-Jobi Induces Cell Cycle Arrest through P53/P21 Signaling Pathway. Biomed Pharmacol J 2016;9(2). Available from: http://biomedpharmajournal.org/?p=7529 |
Introduction
Cancer is a complex disease with multiple genetic and epigenetic changes. Cancer cells proliferate without stopping and spread into surrounding tissues. Chemotherapy is the most common types of cancer treatment. p53 plays an important role in cancer treatment mainly by increasing cell cycle arrest, senescence, DNA damage and apoptosis and interconnecting with many cellular functions. [1], [ 2] Targeting p53 provides many opportunities to fight against cancer. p53 regulates various responses to genotoxic stress by controlling the transcription of numerous target genes involved in cell cycle and DNA repair (p21, GADD45), apoptosis (Bax, Bcl2), and senescence (Sirt1). [3], [ 4] One promising cancer treatment strategy is the development natural products that induce p53 transcription activity and its target genes to induce growth arrest and apoptosis in p53 wild-type cancer cells.
Coix seed (Coix lachryma-jobi) is used in Vietnam and Chinese as a nutritious food and traditional medicine. Previous studies demonstrated that Coix seed extract exhibits anti-inflammatory, protects against oxidative stress, and anti-cancer activities. [5], [6] Coix extract constituents inhibited NF-kB and protein kinase C signalling in cancer cells to suppress growth MDA-MB-231 xenograft in athymic nude mice, [7] inhibited COX-2 expression of human lung cancer cells, [5] activated PI3K/Akt to upregulate the Nrf2, HO-1 expression to protects against oxidative stress-induced injury. [8]
Triolein, one active component in Coix seed extract, exhibits anti-tumor activity. The anti-cancer activities of triolein are limited due to its use as a cell fuel. Triolein is degradated by lipoprotein and fatty acids released are used to provide energy via beta-oxidation. [9] However, mechanism investigations on impacts of triolein on cancer cells are necessary before drug development studies. Therefore, this study was performed to investigate impact of triolein on p53 and its target genes that involves in cell cycle arrest in breast cancer cells. We found that triolein from Coix seed extract induces p53 protein level in concentration-dependent manner in MCF-7 cells. Triolein up-regulated the expression of p53-dependent target genes that related to cell cycle arrest such as p21 and cyclin D1, reducing DNA content. This study provided scientific evidences for the use of Coix seed.
Experimental
Chemicals and Cell Culture
Most chemicals were purchased from Sigma (St. Louis, MO). p53, p21, Bax, p27, Cyclin D1, phospho-Rb, total-Rb, E2F1, anti-mouse and anti-rabbit were obtained from Cell Signaling. MCF-7 cells were obtained from the American type culture collection (Bethesda, MD) and incubated at 37 °C in a 5% CO2/95% air atmosphere in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin.
Western Blot Analysis
After protein determination using BCA protein assay kit (Pierce, Rockford, IL), aliquots of the lysates (10-30 μg of protein) were boiled for 5 min and electrophoresed on 10% SDS-polyacrylamide gels. Proteins in the gels were transferred onto nitrocellulose membranes and then incubated with primary antibodies specified (p53, p21, Bax, p27, Cyclin D1, phospho-Rb, total-Rb, E2F1, β-actin). Bands were visualized using ECL (GE Healthcare Life Sciences) and images were developed using the LAS 3000.
Brdu Assay
BrdU assay was performed as described. [10]
Plant Materials and Extraction
The seeds of Coix lachryma-jobi var. ma-yuen were collected in Yenbai province, Vietnam, in December 2014. The specimen of materials has been identified by one of authors Dr. Do Thi Ha (D.T.H). The specimen (HTV-211214) have been deposited with the Deparment of Phytochemistry, National Institute of Medicinal Materials, Viet Nam.
Sample Preparation for Bioassays
Each sample (adlay aril (CL1), adlay brain (CL2) and adlay seed kernel (CL3), 50 g) was extracted with 96% EtOH (3 × 0.5 L) and then evaporated to dryness under vacuum at 40 ºC to obtain the 96 % CL1 (2.81 %; wt/wt), CL2 (11.64 %; wt/wt), CL3 (11.46 %; wt/wt). Adlay aril (the CL2 extract (5,32 g) was suspended in 100 mL H2O, followed by sequential partitioning with hexane, ethyl acetate, and water, to give CL2H, 2.44 g), ethyl-acetate-soluble (CL2E, 0.1 g), water-soluble (CL2W, 2.78 g) extract. The extracts were stored at -20 ºC until tested.
Extraction and Isolation of Compounds from the Significant Bioactive Fraction
Adlay aril powder (5,85 kg) was extracted three times with 96% ethanol at boiling temperature (20l × 3 h × 3 times). The combined filtrates were evaporated to dryness in vacuo at 40 oC. The residue (681,1 g) was stirred in 1L MeOH and then suspended in 2 l H2O, followed by sequential partitioning with hexane (Hx), ethyl acetate (EtOAc), and water (W), to give hexane-soluble, ethyl-acetate-soluble, and water-soluble fractions. Hexan-soluble fraction (280 g) was then subjected to silica gel (230–400 mesh) column chromatography with successive elution by a Hex/EtOAc gradient (100:0, 70:1,…, 1:10,…) and MeOH solvent system. Subfractions with the same thin-layer chromatography (TLC) patterns were combined into one fraction; thus 9 fractions were obtained (AHH1A~AHH1I). Rechromatography of the first bioactive subfraction AHH1A (80 g) on silica gel columns using Hx-EtOAc gradient (100:0, 50:1, 30:1, 10:1) to yield compound 1 (3,00 g). Fraction T1CL1F (13,6 g) was subjected to silica gel (230–400 mesh) column chromatography, eluted with dichloromethan (DCM) to obtain five sub-fractions T1CL1F1~ T1CL1F5. The fraction T1CL1F5 (1,5 g) was purification by column chromatography with a gradient solvent system DCM-MeOH (50:1, 30:1, 20:1, 10:1) obtained compound 2 (88,8 mg). Compounds 3 (3,0 g) and 4 (190.8 mg) were obtained by further purification of the fraction T1CL1F4 (13,6 g) using normal phase chromatography with a gradient solvent system DCM-MeOH (100:1~20:1) and reverse phase chromatography YMC using a solvent system aceton-H2O (4:1).
CLH1
oil; APCI-MS: m/z: 886,8 [M+H]+ (C57H104O6, M = 885,45). 1H-NMR (500 MHz, CDCl3) δH: 5.34 (6H, m, H-8׳, H-9׳, H-8״, H-9״, H-8״׳, H-9״׳), 5.26 (1H, t, H-2), 4.29 (2H, dd, J = 4.0, 12.0 Hz, H-1), 4.15 (2H, dd, J = 6.0, 12.0 Hz, H-3), 2.31 (6H, t, H-1׳, H-1״, H-1״׳), 2.0 (12H, H-7׳, H-10׳, H-7״, H-10״, H-7״׳, H-10״׳), 1.60 (6H, m, H-2׳, H-2״, H-2״׳), 1.30 (60H, CH2), 0.88 (9H, t, H-17׳, H-17״, H-17״׳). 13C-NMR (500 MHz, CDCl3) δC: 173.3 (C-1׳, C-1״׳), 172.8 (C-1״), 130.0 (C-8׳, C-9׳, C-8״׳, C-9״׳), 129.7 (C-8״, C-9״), 68.9 (C-2), 62.1 (C-1, C-2), 14.1 (C-17׳, C-17״, C-17״׳), 22.7-34.2 (CH2). 1H and 13C-NMR data were consistent with literature values of triolein. [11]
CLH3
A colorless powder; M.p. 290 – 2940C; 1H-NMR (400 MHz, Pyridin-d5) δ: 5.35 (1H, brd, H-6); 3.53 (1H, m, H-3); 1.01 (3H, s, H-19); 0.69 (3H, s, H-20); 0.92 (3H, d, J = 6,8 Hz, H-21); 0.83 (3H, d, J = 1.6 Hz, H-26); 0.81 (3H, brs, H-27); 0.85 (3H, brs, H-29); Glu: 5.07 (1H, d, J = 7.2 Hz, H-1′); 4.59 (1H, dd, J = 2.4; 11.7 Hz, H-6a’); 4.43 (1H, dd, J = 5.7; 11.7 Hz, H-6b’); 4.30 (2H, m, H-3′, 5′); 4.06 (1H, m, H-4′); 3.97 (1H, m, H-2′). 13C-NMR (100 MHz, pyridin-d5) δ: 37.2 (C-1); 31.6 (C-2); 71.7 (C-3); 42.2 (C-4); 140.7 (C-5); 121.7 (C-6); 31.8 (C-7); 31.9 (C-8); 50.0 (C-9); 36.4 (C-10); 21.0 (C-11); 39.7 (C-12); 42.2 (C-13); 56.7 (C-14); 24.3 (C-15); 28.2 (C-16); 55.9 (C-17); 11.8 (C-18); 19.4 (C-19); 36.1 (C-20); 18.7 (C-21); 33.9 (C-22); 25.9 (C-23); 45.8 (C-24); 29.1 (C-25); 19.8 (C-26); 18.9 (C-27); 23.0 (C-28); 11.0 (C-29); Glc: 102.9 (C-1′); 79.0 (C-3′); 78.9 (C-5′); 75.7 (C-2′); 72.1 (C-4′); 63.3 (C-6′). 1H and 13C-NMR data were consistent with literature values of daucosterol. [12]
CLH4
A viscous oil. ACPI pos m/z 842.3 [M+H]+ consistent with the chemical formula C53H92O7; ESI neg 161.0 [M-C18H33O-C29H49O-H]– consistent with a glucose unit C6H10O5– (162.05), m/z 413.3 [M-C18H33O-C6H10O5]– consistent with a sitosteryl unit (413.4); m/z 139.2 [M-C43H73O7]+ consistent with an alkene unit (the =C-C- bond fragmentation at position 8 and 9 broken) (C10H19) of an oleyl unit. 1H-NMR (500 MHz, CDCl3) δH: 5.36 (2H, m, -CH=CH-), 5.34 (1H, m, H-6), 4.44 (1H, dd, J = 5.5, 12.0 Hz, H-6’a), 4.38 (1H, d, J = 7.0 Hz, H-1′), 4.25 (1H, dd, J = 2.0, 12.0 Hz, H-6’b), 3.56 (1H, m, H-5′), 3.54 (1H, dd, 8.0, 8.5 Hz, H-5′), 3.46 (1H, m, H-6’a), 3.44 (1H, m, H-4′), 3.38 (1H, dd, J = 9.0, 11.5 Hz, H-2′), 3.55 (2H, dd, J = 6.0, 11.5 Hz, H-H3b), 2.35 (2H, m, H-2″), 1.28 (30H, m, (CH2)15, 1.09 (3H, s, H-19), 0.98 (3H, d, J = 6.0 Hz, Me-18”), 0.95 (3H, d, J = 6.8 Hz, Me-21), 0.89 (3H, t, J = 7.0 Hz, Me-29), 0.88 (3H, d, J = 7.0 Hz, Me-26), 0.68 (3H, s, Me-18). 13C-NMR (500 MHz, CDCl3) δC: 174.6 (C-1’׳), olefinic carbons: 140.3 (C-5), 130.2 (C-9’׳), 130.0 (C-10”), 121.1 (C-6), 73.5 (C-3), 56.8 (C-14), 56.1 (C-17), 50.2 (C-9), 45.8 (C-23), 42.4 (C-13), 39.8 (C-12), 37,3 (C-1), 36,7 (C-10), 36,1 (C-20), 34,2 (C-22), 31,9 (C-8), 31,8 (C-7), 31,5 (C-2), 29,1 (C-25), 28,3 (C-16), 25,2 (C-23), 24,3 (C-15), 23,1 (C-28), 21,0 (C-11), 19,4 (C-26), 19,3 (C-19), 19.0 (C-27), 18,8 (C-21), 11,8 (C-18), 11,0 (C-29); Glc: 100.2 (C-1′), 79.6 (C-5′), 76.0 (C-3′), 73.9 (C-2′), 70.2 (C-4′), 63.3 (C-6′), 174.6 (C-1″), 33.9 (C-2″), 31.8 (C-8″), 31.7 (C-10″), 29.2-29.7 (-CH2-, C-4″, 5″, 6″, 7″, 12″, 13″, 14″, 15″, 16″), 25.7 (C-3″), 22.7 (C-17″), 14.1 (C-18″). 1H and 13C-NMR data were consistent with literature values of sitosterol-3-O-6-oleoyl-b-D-glucopyranoside. [13]
CLH5
Colorless gum; APCI-MS pos: m/z 355.2 [M+H]+ and ESI pos: m/z 394.1 [M+Na]+ consistent with molecular formular C21H38O4. 1H-NMR (500 MHz, CDCl3) δH: 5.34 (2H, m, H-9׳, H-12׳), 4.08-4.11 (2H, dd, J = 6.0, 11.0 Hz, H-1a, H-1b), 3.87 (1H, m, H-2), 3.63 (1H, dd, 4.5, 11.5 Hz, H-3a), 3.55 (2H, dd, J = 6.0, 11.5 Hz, H-H3b), 2.77 (2H, dd, J = 6.5, 7.0 Hz, H-11′), 2.34 (2H, t, J = 7.5 Hz, H-2׳), 2.00 (4H, H-8׳, H-13׳), 1.62 (2H, m, H-3׳), 1.31 (20H, 10CH2), 0.89 (3H, t, H-18׳). 13C-NMR (500 MHz, CDCl3) δC: 174.6 (C-1׳), olefinic carbons: 130.4 (C-9׳), 130.2 (C-13′), 129.9 (C-10׳), 128.2 (C-12′), 70.1 (C-2), 65.3 (C-1), 63.4 (C-3), 34.2 (C-11′), 32.0 (C-8′, 14′), 31.7 (C-2′), 29.2-29.8 (7 CH2), 27.3 (C-4′), 25.0 (C-3′), 14.1 (C-18׳). 1H and 13C-NMR data were consistent with literature values of 2,3-dihydroxypropyl octadecadienoate (1-monolinolein). [14]
P-values were calculated by Student’s t-test or one-way analysis of variance followed by Bonferroni post-hoc testing using GraphPad Prism 5 (GraphPad Software Inc.). P < 0.05 was considered statistically significant. Data are expressed as mean ± S.E.M. *, p<0.05; **, p<0.01; ***, p<0.001.
Results and Discussion
p53 tumour suppressor protein has been demonstrated to induce apoptosis and cell cycle arrest in cancer cells [1]. To screen natural drug candidates against cancer, we first determined the effect of ethanol 96% extractions from Coix lachryma-jobi includes: adlay aril, adlay brain and adlay seed kernel (1 mg/ml) on p53 protein expression using western blot analysis. Fig 1A indicated that adlay aril extraction most potential increased p53 in MCF-7 cells. From adlay aril extraction, 3 fractions include: n-hexan, ethyl acetate and water were prepared and further examine the effects of these fraction on p53 expression. As shown as in Fig 1B, n-hexan fraction most activated p53 protein expression in MCF-7 cells. From this fraction, we isolated 4 compounds: CLH1 (triolein), CLH3, CLH4 and CLH5. Using western blot analysis, we found triolein (100 μM) compound most significant increase p53 protein expression level in MCF-7 cells (Fig 1C).
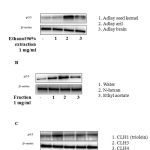 |
Figure 1: Trioelin activates p53 in MCF-7 cells (A) Effect of p53 by 96% ethanol extraction from Coix lacryma-jobi (adlay aril, adlay brain and adlay seed kernel) at 1mg/ml. Cells were incubated with different extractions for 24h. Cells were then harvested and analysis by western blot analysis. (B) Effect of three fraction: n-hexan, ethyl acetate and water from adlay aril extraction on p53 protein expression. (C) Effect of four compounds isolated from n-hexan fraction on p53 protein expression. (n=3)
|
p53 expression up-regulated p21-cell cycle arrest inducer to induce cell cycle arrest and then repair the damaged DNA due to increase apoptosis in cancer cells [15]. Thus, we examine the effect of triolein on p21, p27 and Bax protein expression. As expect, p53, p21, p27 and Bax protein level were significant increased by triolein in dose-dependent (Fig. 2A).
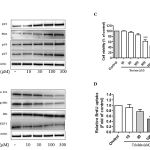 |
Figure 2: (A) Effect of trioelin on p53, p27, Bax and p21 protein expression in dose-dependent manner in MCF-7 cells. Cells were treated with various concentrations of triolein and then cells were harvested to subject to western blot analysis (n=3). (B) Effect of triolein in dose-dependent on Cyclin D1, phospho-Rb, Rb and E2F1 protein expression (n=3). (C) Cell viability after treatment of triolein in MCF-7 cells by MTT assay (n=4). (D) Effect of triolein on DNA synthesis by BrdU assay (n=4). *, p<0.05; **, p<0.01; ***, p<0.001 relative to control.
|
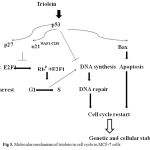 |
Figure 3: Molecular mechanism of triolein in cell cycle in MCF-7 cells.
|
Cyclin D1 is an important regulator in G1/S phase of cell cycle, cell proliferation and apoptosis. E2F1 was reported to induce apoptosis result from the inhibition of Rb. By using western blot analysis, Fig. 2B presented Cyclin D, phospho-Rb and E2F1 were significant suppressed by triolein in dose-dependent manner (10 μM-300 μM). Moreover, we observed the cell cytotoxicity by triolein at 300 μM. p53/p21 plays the important key event in the control of cell cycle, apoptosis and cellular stability (ref). During DNA damage process, wild-typep53 is expressed to increase cell cycle arrest at several checkpoints to repair the damaged DNA. Our study demonstrated that triolein inhibited DNA synthesis in dose-dependent mamner. These data suggest triolein isolated from Coix lacryma-jobi induces cell cycle arrest through p53/p21 signaling pathway
Declaration of Interest
These authors have declared that there is no conflict of interest. This research is funded by Hanoi National University under Tay Bac Program Grant number KHCN-TB.05C/13-18
References
- Levine AJ, Momand J, Finlay CA. Nature 1991 351: 453-456.
- Vogelstein B, Lane D, Levine AJ. Nature 2000 408: 307-310.
- Tokino T, Nakamura Y. Crit. Rev. Oncol. Hematol. 2000 33: 1-6.
- Mirza A, Wu Q, Wang L, McClanahan T, Bishop WR, Gheyas F, Ding W, Hutchins B, Hockenberry T, Kirschmeier P, Greene JR, Liu S. Oncogene 2003.; 22: 3645-3654.
- Hung WC, Chang HC. J. Agric. Food Chem. 2003 51: 7333-7337.
- Chen HH, Chiang W, Chang JY, Chien YL, Lee CK, Liu KJ, Cheng YT, Chen TF, Kuo YH, Kuo CC. J. Agric. Food Chem. 2011 59: 6444-6452.
- Woo JH, Li D, Wilsbach K, Orita H, Coulter J, Tully E, Kwon TK, Xu S, Gabrielson E. Cancer Biol. Ther. 2007 6: 2005-2011.
- Chen HH, Chen YT, Huang YW, Tsai HJ, Kuo CC. Free Radic. Biol. Med. 2012 52: 1054-1066.
- Guardiola-Serrano F, Beteta-Göbel R, Rodríguez-Lorca R, Ibarguren M, López DJ, Terés S, Alvarez R, Alonso-Sande M, Busquets X, Escribá PV. J. Pharmacol. Exp. Ther. 2015 354: 213-224.
- Ki SH, Lee JW, Lim SC, Hien TT, Im JH, Oh WK, Lee MY, Ji YH, Kim YG, Kang KW. Br. J. Pharmacol. 2013 168: 932-945.
- Voutquenne L, Lavaud C, Massiot G, Sevenet T, Hadi HA. Phytochemistry 1999 50: 63-69.
- Soumia M, Hamada H, Catherine L, Christophe L, Mohammed B. Rec. Nat. Prod. 2012 6: 292-295.
- Hashimoto T, Tori M, Asakawa Y. Phytochemistry 1991 30: 2927 – 2931.
- 1 Prinsen P, Gutiérrez A, Faulds CB, del Río JC. J. Agric. Food Chem. 2014 62: 1664-1673.
- Yeh YT, Yeh H, Su SH, Lin JS, Lee KJ, Shyu HW, Chen ZF, Huang SY, Su SJ. Free Radic. Biol. Med. 2014 74:1-13.







