Manuscript accepted on :Aprili 05, 2016
Published online on: --
Plagiarism Check: Yes
Mohsen Mohammadi1, Mohammad Jafar Rezaie2, Ayoob Rostamzadeh3, Azra Allahveisi2, Hamid Reza Mohammadi4, Fatemeh Mohammadi5 and Ardeshir Moayeri6*
1Hepatitis Research Centerand Department of Pharmaceutical Biotechnology, Faculty of Pharmacy, Lorestan University of Medical Sciences, Khorramabad, Iran
2Cellular and Molecular Research Center, Department of Anatomical Sciences, Faculty of Medicine, Kurdistan University of Medical Sciences, Sanandaj, Iran.
3Department of Anatomy and Neuroscience, Faculty of Medicine, Shahrekord University of Medical Sciences, Shahrekord, Iran.
4Department of Toxicology and Pharmacology, School of Pharmacy, Shiraz University of Medical Sciences, Shiraz, Iran
5Brain and Spinal Cord Injury Research Center, Neuroscience Institute, Tehran University of Medical Sciences, Tehran, Iran.
6Department of Anatomy, Faculty of Medicine, Ilam University of Medical Sciences, Ilam, Iran.
*Corresponding Author E-mail: moayeri46@medilam.ac.ir
DOI : https://dx.doi.org/10.13005/bpj/972
Abstract
Melatonin (MT) (N-acetyl-5-methoxytryptamine) is a lipophilic and hydrophilic indoleamine with various physiologic functions MT presents in all cells and tissues and distributes in all cell compartments. Liver is the only organ of circulating MT metabolism. The roles of MT in various liver pathologies have been extensively studied, and it is believed that oxidative stress (OS) and lipid peroxidation (LPO) are the key causing factors of almost all conditions compromising liver function, including nonalcoholic fatty liver disease (NAFLD), nonalcoholic steatohepatitis (NASH), diabetes, liver fibrosis and cirrhosis. MT improves energy production and reduces apoptosis OS and LPO in liver. MT protects liver against OS through multiple ways: inhibition of inflammatory cytokines, direct scavenge of free radicals, stimulation of antioxidant enzymes, decrease of mitochondrial electron leakage and synergistic function with other classical antioxidants.
Keywords
Liver disease; Melatonin; Free radical; Reactive oxygen species
Download this article as:| Copy the following to cite this article: Mohammadi M, Rezaie M. J, Rostamzadeh A, Allahveisi A, Mohammadi H. R, Mohammadi F, Moayeri A. Signaling Pathways of Melatonin in Prevention of Liver Disorders via Suppressing of Oxidative Stress in Cellular Level. Biomed Pharmacol J 2016;9(2). |
| Copy the following to cite this URL: Mohammadi M, Rezaie M. J, Rostamzadeh A, Allahveisi A, Mohammadi H. R, Mohammadi F, Moayeri A. Signaling Pathways of Melatonin in Prevention of Liver Disorders via Suppressing of Oxidative Stress in Cellular Level. Biomed Pharmacol J 2016;9(2). Available from: http://biomedpharmajournal.org/?p=7675 |
Introduction
Melatonin and Liver Function
Melatonin (MT) (N-acetyl-5-methoxytryptamine) is a derivative of tryptophanthrough the serotonin pathway, and synthesized or present in variety of organs, such as pineal gland and gastrointestinal tract(1-3). MT is a highly lipophilic and quite hydrophilicindoleamine product. MT is capable of interacting with lipid bilayers, crossing biological membranes and reaching all cellular compartments due to its high lipophilicity and rather small size(1, 4). MT is serves as an autacoid factor with amphiphilicity and restricted toxicity even at high concentrations of administration. MT has the maximum secretion levelsduring the dark period of light/dark cycle, and the diurnal fluctuation of this hormone is reflected in its plasma level(5, 6).MT initially found to control circadian, and has functional interactions with the circadian system and the neuroendocrine axis. Besides its multi-faceted free radical scavenging function, MTacts asantioxidant, gene regulator, immunomodulator, anti-apoptotic, anti-inflammatory, anti-gonadotropic, cytoprotective and oncostaticagent as well as athermoregulator hormone (2,6)(2, 6). Target cells have membrane receptors for MT. Circadian control and antioxidant functions of this hormone are applied in a receptor-mediated manner. There are three receptors for MT: MT1 and MT2, which control various processesincluding cell proliferation, arterial vasoconstriction and metabolic functions, and also MT3, probably involves in cellular redox status regulation(1, 7, 8).By using 2-1251-iodomelatonin (1251-aMT), cell nuclei proteins were identified to be in association with MT; 2-1251-aMT has the high affinity in binding mouse hepatocyte membrane preparations. Nucleus binding sites may be a physiological MT receptor and the target for intracellular function of MT(2, 7). The MT receptor type on mouse hepatocyte is of MT1 subclass, specifically MT1b. Local secretion of MT seems to modulate the action of gastrointestinal system, including liver, and that MT probably acts as a mediator in inter-organ communication between liver and gut(4, 9, 10).Liver is one of the organs in the body for having high activities of antioxidant enzymes, and it is the solely organ in metabolism of circulating MT that by in turn regulates liver metabolismof carbohydrates and lipids.MT preserves glycogen stores, improves liver fatty changes, modulates liver concentrations of glucagon and insulin receptors, attenuates plasma cholesterol levels, induces gap junction expression and inhibits hepatocyte proliferation; inhibition of hepatocyte proliferation is to protect these cells against oxidative stress (OS)(2, 8, 11). MT metabolites still have antioxidant activity, as 6-hydroxymelatonin sulfate scavenges free radicals.MT concentration in gastrointestinal tract is several hundred times more than its level in pineal gland(2, 10, 12). MT has the highest concentration in the hepatobiliary system, namely liver, portal circulation and bile, where its level in bile is 1000-fold higher than blood levels during daytime and the concentration of MT in bile is 6 times higher than the concentration in tissue (5, 7, 12).
MT Distribution and Metabolism in Liver
MT has the significant higher content in the gut than in the pineal gland.MT has the nocturnal pattern of secretion over 8 h, and itspharmacological doses have no significant side effects, as 1200 mg/kg of MT has been used in human subjects without sings of toxicity(8, 11, 13). Levels of MT are associated with various disorders and metabolic disturbances. MT has the highest level in serum. Serum MT concentrations are reduced by continuous light exposure(11, 14). Serum levels of MT in mice oscillate from 160 pm per day to 1800 pm per night, which represents about 32-360 pg/ml. In human plasma levels of MT during the night is exceeded 55 pg/ml, and in the morning hours is in the range of 20 pg/ml. Daily production of normal MT in human, male subjects, is 28.8 µg, and daily excretion of MT from the bile into the intestinal tract in human subjects is about 51 ng(3, 12, 15). Increased systemic levels of MT has been documented following tryptophan administration in rats, pigs and cheeks, and increased plasma levels of MT has been attested following food restriction in human subjects.In humans total serum antioxidative capability correlates well with serum MT levels(2, 13).In human the peak MT serum level after administration of 2 mg of MT is around 1-2 hours and after administration of 80 mg it rose from 350 to 1000 times over its concentration during night-time. Dietary MT results in a notable increase in MT levels, and has the greatest effects in middle-aged mice(1). MT is metabolically degraded at the site of production, and circulating MT in metabolically degraded in liver(2).When oxidized, MT is not easily reduced and is not implicated in regenerating processes which may give rise to toxic recycling and free radicals formation(3). Instead, MT, upon oxidation, converts to antioxidant compounds, such as N1-acetyl-5-methoxykynuramine, N1-acetyl-N2-formyl-5-methoxy-kynuramine(AFMK) and cyclic 3-hydroxymelatonin (4). Cytochrome c, reactive oxygen species (ROS) and myeloperoxidase (MPO) could mediate MT oxidation to AFMK; degradation of AFMK to N1-acetyl-5-methoxykynuramine (AMK) is carried out by both arylamineformamidase and catalase (CAT)(2).Semak et al indicated that microsomal cytochrome P450s are involved in conversion of MT into O-demethylation and hydroxylation at positions 2 and 6 as well as AFMK, and mitochondrial cytochrome P450s are involved in conversion of MT into 2-hydroxymelatonin, N-acetylserotonin, AFMK, 6-hydroxymelatonin. They also showed that, in comparison with microsomal enzymes, mitochondrial cytochrome P450s have higher affinity for MTO-demethylation and 6-hydroxylation(2). 6-hydroxylation is mainly mediated by cytochrome P4501A2 (CYP1A2), a high-affinity enzyme for MT metabolism in human liver microsome(5).
Role of MT in Energy Production at Liver
Adequate maintenance ofAdenosine triphosphate(ATP) levels is crucial for the liver cells viability, and its depletion causes membrane permeability derangement contributing to irreversible cell injury(6).The depletion of ATP during ischemia leads to a loss of the transmembrane potential of mitochondria which is associated with overload of Ca2+ and the following mitochondrial permeability transition (MPT) pore opening. Then overload of ions and hyperosmotic effect cause rapid swelling and rupture of mitochondria; as a result, cytochrome c, at the intermembrane space, releases into the cytosol and activates caspase 9 which in turn activates caspase 3 and the subsequent DNA fragmentation (7). Release of cytochrome c may also due to production of NO triggered by increased Ca2+ levels. Furthermore, ONOO- and possibly NO inhibit respiration through suppression of aconitase, a component of the Krebs cycle, in both mitochondria and cytosol (8).Mitochondria are organelles in which high concentrations of MT seem to accumulate (9, 10). Concentration of MT in the mitochondria is 100 times higher than in the blood (2). MT (10 mg/kg BW) improves hepatic injury metabolism by reduction of the rate of mitochondrial energy transfer suppression and restoration to near normal of mitochondrial structure (11).MT (1 nM) increases the activity of oxidative phosphorylation and complexes I&IV in rat liver mitochondria resulting in production of ATP (12). The capability of MT to donate and accept electrons causes improvement of respiratory chain activity and increase of electron flow (6, 13).MT could also detoxify electron-deficient ROS through its electron donation ability. Furthermore, MT reduces electron leakage and free radicals generation via potentiation of mitochondrial electron transport chain efficiency (9). This efficiency is provided through stabilization of mitochondrial inner membranes by MT(14). Glutamate dehydrogenase (GDH) activity is a marker of the integrity of mitochondrial membrane. Administration of MT (10 mg/kg) 15 min before ischemia and immediately before reperfusion increases the activity of GDH (7).
Prevention of OS and LPO in Liver by MT
OS is the consequence of redux balance disruption (10) between antioxidant and pro-oxidant species leading to oxidative damage of cellular macromolecules(15) and is the causing factor of aging (10). These pro-oxidants are collectively known as ROS(15). Approximately, 1-2% of the consumed oxygen by the organism at physiological level is converted to ROS (4), and under normal conditions, antioxidant defense systems keep ROS at physiologically optimal levels(16).Different types of ROS are considered as important mediators in various models of tissue injury (17), such as toxic reactions, inflammation and ischemic processes (18).The excessiveproduction of ROS results in oxidative destruction to the deoxyribonucleic acid (DNA), proteins, and lipids. ROS can act with the nucleic acids attacking the nitrogenous bases and the sugar phosphate backbone and can elicit single- and double-stranded DNA breaks. ROS also attack structural and enzymatic proteins by the oxidation of residual amino acids, prosthetic groups, formation of cross links, protein aggregates, and proteolysis. The inactivation of the key proteins can lead to the serious consequences in the vital metabolic pathways. Lipid peroxidation (autooxidation) is a process of oxidation of polyunsaturated fatty acids due to the presence of several double bonds in their structure and it involves production of peroxides (chemical compounds in which two oxygen atoms are linked together by a single covalent bond), ROS, and other reactive organic free radicals(19).In fatty liver, the predominant pro-oxidants are superoxide anion (O2•–) radical, singlet oxygen, •OH and H2O2 (15); ROS are involved in damaging various essential biological molecules including membrane lipids, DNA and cellular proteins (3). ROS are derived from mitochondrial, microsomal and other hepatocellular pro-oxidant pathways in the critical reduction of antioxidant defenses (15).
A major source of ROS is from the mitochondrial electron transport chain. ROS may be produced by other mechanisms. Radical scavenging function of MT exerts at both pharmacological and physiological concentrations and in vitro and in vivo(6). MT exerts free radical scavenging by having acetyl group on the side chain and methoxyl group at the position five(20). MT scavenges reactive nitrogen, reactive oxygen species (ROS) (15), nitric oxide (NO) and singlet oxygenas well as is able to repair oxidatively damaged membrane lipids and cytosolic proteins (21).MT is also able to decrease oxygen consumption in liver mitochondria, which results in less ROS production (22).ROS production resulting in oxidative damage increases intracellular Ca2+ levels(23). Ca2+ then enters the nucleus to activate nucleases which cause breaks in DNA strands which to inhibited influx of Ca2+ in cells via MT(23).NF-kB activation, protein carbonyls and TBARS are OS markers (24). NF-kB signaling activation is central to the inflammatory response pathophysiology(9). NF-kB regulates various inflammatory genes including iNOS, IL-6, tumor necrosis factor-α (TNF-α) and IL-1β (9). Although IL-1 does not induce liver damage, it could stimulate inflammatory cells to produce more TNF-α, IL-6 and IL-8 cytokines (21).Liver is one of the organs vulnerable to the attack of pro-inflammatory cytokines (25).Liver cells that produce pro-inflammatory cytokines are Kupffer cells, HSCs, BECs and endothelial sinusoid cells (25). Kupffer cells play key roles in phagocytosis, immunomodulation and biochemical attack. These cells are activated by damaged hepatocytes, infiltrating inflammatory cells and toxic metabolic agents (21). NF-kB is inhibits by Nuclear erythroid 2-related factor 2 (Nrf2)(9).Nrf2 is a chief transcription factor involves in regulation of cellular antioxidative response against ROS. By binding to antioxidant response (ARE), upon cell stimulation, Nrf2 causes upregulation of antioxidant enzymes, and decreases sensitivity of the cells to OS(9, 24). ARE sequence exists in the promoter region of most of the antioxidant enzymes encoding genes. Antioxidant enzymes gene expression regulates by Nrf2/ARE are HO-1, quinone oxidoreductase-1 (NQO1), GST and SOD2. Nrf2 modulates acute inflammatory responses in which Nrf2-deficient mice show increased activation of NF-kB in response to conditions like LPS. Nrf2 also plays a crucial role in the cellular redox balance maintenance (9).MT has immunomodulatory action, and by inhibition of nuclear translocation of NF-kB and its further binding to DNA may take part in the decrease of pro-inflammatory cytokines and adhesion molecules(24)(Figure 1).MT also increases the nuclear expression of Nrf2(9) and alters redox balance through increase of GSH levels and scavenge of ROS (26). Wang et al. showed that MT has no direct effect on suppression of Kupffer cells (21).Cell response against oxidative effects conveys through production of heat shock proteins (HSP), metallothionein(27) and antioxidant enzymes (3).HSPs are molecular chaperons with key roles in the cell protection against OS toxic effects. MT is a cytosolic protein enriched in cysteine which acts as a potent ROS scavenger, and protects the cells against highly reactive compounds; in defense mechanisms to toxicity, MT is regarded as a cellular detoxification mediator of metals (27).SOD and CAT are important antioxidant enzymes and have key defensive roles against free radicals (3)so that cooperative action of intracellular SOD, CAT and GPx is involved in liver detoxification of ROS (4). Other antioxidant enzymes are reduced GSH, GPx (13)(13), glutathione reductase/GRx, glucose 6-phosphate dehydrogenase, γ-glutamylcysteine synthase (4). Glutathione peroxidase (GPx), as the most abundant peroxidase (28), is an antioxidant enzyme involving in metabolism of potentially damaging molecules into non-toxic products (11). Reduced GSH along with GPx are served as the main antioxidant protection of mitochondria, the organelle with lack of CAT for protection; mitochondria obtain GSH from cytosol. Chemical-induced oxidative liver injury showed depletion of GSH in mitochondria, rather than cytosol, for irreversible cellular damage development, therefore, mitochondrial GSH homeostasis is crucial for defending mitochondria and thus the whole cell against oxidative damage (13). GSH content is an index for the cellular redox state (7) in which oxidized and reduced GSH ratio (GSSG/GSH) is a sensitive cellular redox state indicator (16).GSH plays a main role in the regulation of intracellular ROS (26).GPxand CAT reduce the levels of hydrogen peroxide (H2O2)(28). GPx also reduces lipid peroxide, and it is important in LPO prevention and maintenance of the function and structure of biological membranes (15). MT scavenges H2O2, thereby preventing the consumption of the GSH pool within mitochondria and the subsequent mitochondrial damage (29).MT protects mitochondria from oxidative injury through decrease of excess Krebs’ cycle, thereby restricts overstimulation of cellular respiration (22).MTis also well-known to regulate and/or maintain the intracellular GSH concentration (10) and promotes GSSG to GSH conversion (30).It has also been reported by Albarran et al. that there is a presumable relation between the MT rhythm with night time increase of SOD activity and blood levels of antioxidants (31).However, depending upon the incubation time and concentration, MT could exert both antioxidant and pro-oxidant features; to explain, using MT at concentrations of 0.1-10 µm in the human liver cell line (HepG2) for 24 h shows antioxidant properties through increase of GSH level, which associated with cell viability improvement.
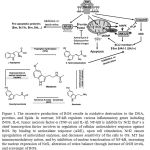 |
Figure 1: The excessive production of ROS results in oxidative destruction to the DNA, proteins, and lipids. |
In contrast, NF-kB regulates various inflammatory genes including iNOS, IL-6, tumor necrosis factor-α (TNF-α) and IL-1β. NF-kB is inhibits by Nrf2 that’s a chief transcription factor involves in regulation of cellular antioxidative response against ROS. By binding to antioxidant response (ARE), upon cell stimulation, Nrf2 causes upregulation of antioxidant enzymes, and decreases sensitivity of the cells to OS. MT has immunomodulatory action, and by inhibition of nuclear translocation of NF-kB, increasing the nuclear expression of Nrf2, alteration of redox balance through increase of GSH levels, and scavenger of ROS.
Conclusion
OS is an inducer of hepatocyte apoptosis, which works through intrinsic apoptosis pathway. OS and LPO potentially increases mitochondrial membrane permeability resulting in mitochondrial integrity loss and further cytochrome c release into cytoplasm; then, cytochrome c joins Apaf-1 which in turn polymerizes into apoptosome. Apoptosis can promote by ROS generation increment or endogenous antioxidant depletion. Liver mitochondria of mice showed age-related reduction in activities of complex I and IV, leading to excessive free radical generation and less effective mitochondrial defense against oxidative damage. Melatonin normalizes cytochrome c content in both cytosol and mitochondria. Melatonin also increases bcl2 antiapoptotic levels and decreases Baxantiapoptotic levels. The Bcl2 family is a key regulator of the intrinsic apoptosis pathway, which includes both antiapoptotic (bcl2 or bcl-xL) and pro-apoptotic (Bax) proteins. Melatonin is the antagonist of the apoptosis intrinsic pathway by alteration of Bax/bcl2 levels mostly through its radical scavenging function. Furthermore, melatonin-reperfused livers increase the area of ROS-positive hepatocytes; interestingly, this increase of ROS production did not augment OS to the liver parenchyma; instead, it was in agreement with the melatonin increase of bile production and ATP levels. OS induction, antioxidant status depletion and mitochondrial dysfunction are the relevant features in liver diseases.Moreover, Nrf2 that is a chief transcription factor involves in regulation of cellular antioxidative response against ROS causes upregulation of antioxidant enzymes, and decreases sensitivity of the cells to OS.
References
- Lahiri DK, Ge YW, Sharman EH, Bondy SC. Age‐related changes in serum melatonin in mice: higher levels of combined melatonin and 6‐hydroxymelatonin sulfate in the cerebral cortex than serum, heart, liver and kidney tissues. Journal of pineal research. 2004;36(4):217-23.
- Semak I, Korik E, Antonova M, Wortsman J, Slominski A. Metabolism of melatonin by cytochrome P450s in rat liver mitochondria and microsomes. Journal of pineal research. 2008;45(4):515-23.
- Tang L, Reiter RJ, Li Z-R, Ortiz GG, Yu BP, Garcia JJ. Melatonin reduces the increase in 8-hydroxy-deoxyguanosine levels in the brain and liver of kainic acid-treated rats. Molecular and cellular biochemistry. 1998;178(1-2):299-303.
- Subramanian P, Mirunalini S, Pandi-Perumal SR, Trakht I, Cardinali D. Melatonin treatment improves the antioxidant status and decreases lipid content in brain and liver of rats. European journal of pharmacology. 2007;571(2):116-9.
- Facciolá G, Hidestrand M, von Bahr C, Tybring G. Cytochrome P 450 isoforms involved in melatonin metabolism in human liver microsomes. European journal of clinical pharmacology. 2001;56(12):881-8.
- Vairetti M, Ferrigno A, Bertone R, Rizzo V, Richelmi P, Bertè F, et al. Exogenous melatonin enhances bile flow and ATP levels after cold storage and reperfusion in rat liver: implications for liver transplantation. Journal of pineal research. 2005;38(4):223-30.
- Kim SH, Lee SM. Cytoprotective effects of melatonin against necrosis and apoptosis induced by ischemia/reperfusion injury in rat liver. Journal of pineal research. 2008;44(2):165-71.
- Kireev R, Tresguerres A, Vara E, Ariznavarreta C, Tresguerres J. Effect of chronic treatments with GH, melatonin, estrogens, and phytoestrogens on oxidative stress parameters in liver from aged female rats. Biogerontology. 2007;8(5):469-82.
- Jung KH, Hong SW, Zheng HM, Lee DH, Hong SS. Melatonin downregulates nuclear erythroid 2‐related factor 2 and nuclear factor‐kappaB during prevention of oxidative liver injury in a dimethylnitrosamine model. Journal of pineal research. 2009;47(2):173-83.
- Molpeceres V, Mauriz JL, García-Mediavilla MV, González P, Barrio JP, González-Gallego J. Melatonin is able to reduce the apoptotic liver changes induced by aging via inhibition of the intrinsic pathway of apoptosis. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2007;62(7):687-95.
- Petrosillo G, Di Venosa N, Pistolese M, Casanova G, Tiravanti E, Colantuono G, et al. Protective effect of melatonin against mitochondrial dysfunction associated with cardiac ischemia-reperfusion: role of cardiolipin. The FASEB journal. 2006;20(2):269-76.
- Martı́n M, Macıas M, León J, Escames G, Khaldy H, Acuña-Castroviejo D. Melatonin increases the activity of the oxidative phosphorylation enzymes and the production of ATP in rat brain and liver mitochondria. The international journal of biochemistry & cell biology. 2002;34(4):348-57.
- Martín M, Macías M, Escames G, León J, Acuña-Castroviejo D. Melatonin but not vitamins C and E maintains glutathione homeostasis in t-butyl hydroperoxide-induced mitochondrial oxidative stress. The FASEB journal. 2000;14(12):1677-9.
- Catalá A, Zvara Á, Puskás LG, Kitajka K. Melatonin‐induced gene expression changes and its preventive effects on adriamycin‐induced lipid peroxidation in rat liver. Journal of pineal research. 2007;42(1):43-9.
- Pan M, Song YL, Xu JM, Gan HZ. Melatonin ameliorates nonalcoholic fatty liver induced by high‐fat diet in rats. Journal of pineal research. 2006;41(1):79-84.
- Mauriz JL, Molpeceres V, García‐Mediavilla MV, González P, Barrio JP, González‐Gallego J. Melatonin prevents oxidative stress and changes in antioxidant enzyme expression and activity in the liver of aging rats. Journal of pineal research. 2007;42(3):222-30.
- Sewerynek E, Melchiorri D, Chen L, Reiter RJ. Melatonin reduces both basal and bacterial lipopolysaccharide-induced lipid peroxidation in vitro. Free Radical Biology and Medicine. 1995;19(6):903-9.
- Zavodnik L, Zavodnik I, Lapshina E, Belonovskaya E, Martinchik D, Kravchuk R, et al. Protective effects of melatonin against carbon tetrachloride hepatotoxicity in rats. Cell biochemistry and function. 2005;23(5):353-9.
- Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organization Journal. 2012;5(1):1.
- El‐Missiry MA. Prophylactic effect of melatonin on lead‐induced inhibition of heme biosynthesis and deterioration of antioxidant systems in male rats. Journal of biochemical and molecular toxicology. 2000;14(1):57-62.
- Wang H, Wei W, Shen Y-X, Dong C, Zhang L-L, Wang N-P, et al. Protective effect of melatonin against liver injury in mice induced by Bacillus Calmette-Guerin plus lipopolysaccharide. World journal of gastroenterology. 2004;10(18):2690-6.
- Reyes-Toso C, Rebagliati I, Ricci C, Linares L, Albornoz L, Cardinali D, et al. Effect of melatonin treatment on oxygen consumption by rat liver mitochondria. Amino Acids. 2006;31(3):299-302.
- Meki A-RM, Abdel-Ghaffar SK, El-Gibaly I. Aflatoxin B1 induces apoptosis in rat liver: protective effect of melatonin. Neuroendocrinology Letters. 2001;22(6):417-26.
- Bruck R, Aeed H, Avni Y, Shirin H, Matas Z, Shahmurov M, et al. Melatonin inhibits nuclear factor kappa B activation and oxidative stress and protects against thioacetamide induced liver damage in rats. Journal of hepatology. 2004;40(1):86-93.
- Kireev R, Tresguerres A, Garcia C, Ariznavarreta C, Vara E, Tresguerres JA. Melatonin is able to prevent the liver of old castrated female rats from oxidative and pro‐inflammatory damage. Journal of pineal research. 2008;45(4):394-402.
- El-Sokkary GH, Nafady AA, Shabash EH. Melatonin administration ameliorates cadmium-induced oxidative stress and morphological changes in the liver of rat. Ecotoxicology and environmental safety. 2010;73(3):456-63.
- Rezzani R, Buffoli B, Rodella L, Stacchiotti A, Bianchi R. Protective role of melatonin in cyclosporine A-induced oxidative stress in rat liver. International immunopharmacology. 2005;5(9):1397-405.
- Rodriguez C, Mayo JC, Sainz RM, Antolin I, Herrera F, Martin V, et al. Regulation of antioxidant enzymes: a significant role for melatonin. Journal of pineal research. 2004;36(1):1-9.
- FREITAS I, BERTONE V, GUARNASCHELLI C, FERRIGNO A, BONCOMPAGNI E, RIZZO V, et al. In situ demonstration of improvement of liver mitochondria function by melatonin after cold ischemia. in vivo. 2006;20(2):229-37.
- Rapozzi V, Comelli M, Mavelli I, Sentjurc M, Schara M, Perissin L, et al. Melatonin and oxidative damage in mice liver induced by the prooxidant antitumor drug, adriamycin. in vivo. 1999;13(1):45-50.
- Albarran M, Lopez‐Burillo S, Pablos M, Reiter R, Agapito M. Endogenous rhythms of melatonin, total antioxidant status and superoxide dismutase activity in several tissues of chick and their inhibition by light. Journal of pineal research. 2001;30(4):227-33.







