Maid Priyanka Prakash*,P. Vengal Rao , R. V. Venkataramanan , V. Chitra and M. Sumithra
Department of Pharmacology, SRM College of Pharmacy, SRM University, Kattankulathur, India.
DOI : https://dx.doi.org/10.13005/bpj/992
Abstract
Oxidative stress is implicated as one of the primary factor that contributes to the neurodegerative disease, brain damage, stroke, hypoxia etc. The aim and objective of the present study is to investigate the neuroprotective effect of hydrolcoholic seed extract of Langenaria siceraria on hypoxic neurotoxicity induced in wistar rats. The animals were divided into five groups of 8 animals each. Hypoxic neuronal damage was induced by the administration of sodium nitrite 30mg/kg p.o for 14 days. The hydroalcoholic extract (HAE) of L. siceraria was administered at doses 200mg/kg, 400mg/kg b.w, p.o for 14 days. Alteplase 0.9mg/kg i.v was used as standard. Alteration in neurological behavior (Beam walking, Cylinder test, Adhesion test) was performed; changes in various biochemical and antioxidant levels were estimated. The drug treated group showed normal neurological behavior comparable with that of normal control group. The level of Glutathione, Dopamine was reduced and level of TBAR's and nitrates was increased in disease control. The drug treated groups showed elevated levels of Glutathione, Dopamine, and reduced levels of TBAR's and Nitrates in dose dependent manner.
Keywords
Langenaria Siceraria; Oxidative stress; Neurological behavior; Hypoxia; Neurotoxicity
Download this article as:| Copy the following to cite this article: Prakash M. P, Rao P. V, Venkataramanan R. V, Chitra V, Sumithra M. Neuroprotective Effect of Hydroalcoholic Seed Extract of Langenaria Siceraria (Mol) Standl. on Hypoxia Neurotoxicity Induced in Wistar Rats. Biomed Pharmacol J 2016;9(2). |
| Copy the following to cite this URL: Prakash M. P, Rao P. V, Venkataramanan R. V, Chitra V, Sumithra M. Neuroprotective Effect of Hydroalcoholic Seed Extract of Langenaria Siceraria (Mol) Standl. on Hypoxia Neurotoxicity Induced in Wistar Rats. Biomed Pharmacol J 2016;9(2). Available from: http://biomedpharmajournal.org/?p=7512 |
Introduction
Cerebral stroke is a sudden and dramatic development of focal neurologic deficit, varying from trivial neurologic disorder to hemiplegia and coma [1][2]. Stroke occurs when blood flow to the brain is interrupted by either a blocked or burst artery, resulting in a sudden decreased in the blood flow to an area of the brain, depriving brain cells from oxygen and other nutrients, developing ischemia within minutes. Irreversible cell damage occurs for brain cells at the center of the ischemic region, while the ischemia in the surrounding region is incomplete because of blood perfusion from collateral vessels, the region is called as penumbra [3]. Incidence of stroke is approx 200,000 per year. Stroke mortality accounts for 20-30% irrespective of all therapeutics efforts. It is the third leading mortality cause of death in the world. [4][5]
Materials and Methods
Plant material
The Seeds of Lagenaria siceraria was collected from natural habitat in and around Nellore, Andhra Pradesh. The plant is authenticated by Prof. P. Jayaraman, Plant Anatomy Research Center, Medicinal Plant Research unit, Tambaram, Chennai. Regd no. PARC/2012/1411.
Preparation of plant extract
200 g of dried seeds of Lagenaria siceraria was reduced to fine powder and was subjected to successive hot percolation extraction (soxhlet) with hydro alcohol (1:1). After the extraction, the extract was concentrated on water bath.[6]
Preparation of plant extract
200 g of dried seeds of Lagenaria siceraria was reduced to fine powder and was subjected to successive hot percolation extraction (soxhlet) with hydro alcohol (1:1). After the extraction, the extract was concentrated on water bath.[6]
Phytochemical analysis
The Hydroalcoholic extract was subjected to the phytochemical analysis using conventional protocol like alkaloids, flavonoids, carbohydrates, glycosides, saponins, proteins, amino acids, fixed oils, mucilage, etc.[7]
Grouping
Inbreed strains of Wistar rats of either sex weighing 150-200g was taken for the study. The animals were maintained in propylene cages at room temperature and standard 12h day/night cycle. The animals was fed with standard rodent pellet diet and water ad libitum .IAEC proposal no. IAEC 149/2012.
Group I- Control saline p.o
Group II- Sodium Nitrite, 30mg/kg (p.o) for 14 days.
Group III- HAE of L.siceraria + Sodium nitrite, 200mg/kg+30mg/kg (p.o) for 14 days.
Group IV- HAE of L.siceraria + Sodium nitrite, 400mg/kg+30mg/kg (p.o) for 14 days.
Group V- Alteplase + Sodium nitrite, 0.9mg/kg+30mg/kg (i.v+p.o) for 14 days.
Induction of hypoxic neurotoxicity
Hypoxia was induced by administration of sodium nitrite drinking water (sodium nitrite 30 mg/kg dissolved in normal water) by gavages (5 ml/kg dosing volumes) for 14 days except the control group, which was provided with normal water.[8]
Neurological behavior
Beam walking Test
All the animals was placed individually on long beam of 60 cm long and the animals were allowed to walk. The distance and the time taken to walk across 60 cm long beam of 1.2 cm square diameter and a round 1cm diameter, suspended 60 cm over the bench was recorded for each animals.[9]
Cylinder Test
The method of Schallert and colleagues was used with minor modifications. Animals were placed individually in a glass cylinder of 12 cm diameter, which allows rat to stand comfortably on the base with only 1-2 cm in front and behind thus encourages rearing. The number of times the rat reared, and the fore paw used to make first contact in 2 min was recorded.[9]
Adhesive Test
The test was performed in the home cage of the rat, all the animals from the home cage was removed out and kept separately and the bedding materials from the home cage was removed. Small adhesive stickers were placed onto the front paws of the rat, and the duration of time taken to remove it was recorded. This test was repeated twice on each occasion and the mean of both scores was used in the analysis. The rat were given a maximum of 3 min to perform this task.[9]
Biochemical estimation
Estimation of Dopamine
To the separated aqueous phase 0.01ml of 0.4 M hydrochloride acid and 0.01 ml EDTA/sodium acetate buffer (pH 6.9) was added, followed by 0.01 ml iodine solution (0.1 m in ethanol). After 2 min reaction was stopped by addition of 0.01 ml Na2SO3 in 5M NaOH (0.5g Na2SO3 ¬in 2 ml H2O + 18 ml 5M NaOH). Acetic acid (0.01 ml, 10M) was added 1.5 min later, heating the solution to 100°C for 6min. When sample reached room temperature, excitation and emission spectra were read i the micro cuvette. The readings were taken at 330-375 nm.[11]
Estimation of Glutathione
The assay of GSH was determined by the method described by Moron et al. (1979). One milliliter of tissue homogenate (supernatant) and 1.0 ml 20% TCA were mixed and centrifuged at 2500 rpm for 15min at 4°C. In 0.25 ml of supernatant, 2ml of DTNB (0.6) reagent was added. The final volume was made upto 3ml with phosphate buffer (pH 8.0). The colour developed was read at 412 nm against reagent blank. Different concentrations (10-50 µg) of standard glutathione were processed as mentioned above for constructing standard curve. The amount of reduced glutathione was expressed as µg of GSH/mg of protein. [8]
Estimation of Nitrates
Nitrites concentration in the plasma/brain was determined as nitrites by using Griss reagent 400µl of plasma will be mixed with equal volumes of griss reagent and the optical density was determined at 540 nm. Caliberation curve was generated using 0.1 sodium nitrite as standard. The nitrites level in plasma was expressed as µg/ml.[10][12]
Estimation of TBAR’s
Tissue homogenate supernatant (brain)-0.5 ml, 8% sodium dodecyl sulphate (SDS)-0.2ml, 20% acetic acid solution 1.5ml, adjust pH-3.5 with 1N NaOH/0.1Hcl, Thiobarbituric acid, 1.5ml . the incubation mixture was made up to 5ml with double distilled water and them heated in boiling water bath for 30 mins after cooling the red chromogen was extracted in to 5 ml of the mixture of n-butanol and pyridine centrifuged at 4000rpm for 10 min the organic layer was taken and absorbance was measured at 532nm, 1,1,3,3 tetra ethoxy propane is used as an external standard.[13]
Histological study
Rats were euthanized by thiopental sodium (45 mg/kg, i.p) and isolated brain was stored in formaldehyde solution. After dehydration in phosphate buffer 25% sucrose solution coronal cryosection (25m) were cut and stained with hematoxylin and eosin for histophathological studies. [14]
Statistical Analysis
the results was expressed as Mean ± SEM. the data was analyzed by using one way analysis of variance (ANOVA) followed by Dunnett’s test.
Results and Discussion
Beam walking
Animals of all groups were subjected to beam walking test on day 14 of drug treatment. The HAS extract of L.siceraria (200mg/kg,400mg/kg) treated groups showed significant decrease in beam walking time in dose dependant manner when compared with disease control group as shown in table I.
Table 1 :Effect of Hae Siceraria on Beam Walking Test
| Groups | Treatment | Beam walking time (sec) |
| Group I | Control (Normal Saline) | 5.50±0.189 |
| Group II | Sodium Nitrite | 23.50±0.378 a** |
| Group III | HAE of L.siceraria +Sodium Nitrite | 18.75±0.453 b* |
| Group IV | HAE of L.siceraria +Sodium Nitrite | 14.88±0.291 b** |
| Group V | Alteplase+Sodium Nitrite | 13.0±0.378b** |
Values are given as Mean ±SEM for n=8 in each group, comparison were made between a) Group I and Group II b) Group II with Group III, Group IV, Group V* symbol statistical significance done by one way ANOVA followed by Dunnett’s test P<0.01.
Cylinder Test
Animals of all groups were subjected to cylinder test on day 14 of drug treatment. The HAE of L.siceraria (200mg/kg,400mg/kg) treated groups showed significant increase in number of rearing when compared with disease control group as shown in table II.
Table 2: Effect of HAE siceraria on Cylinder test
| Groups | Treatment | Cylinder Test Rearing |
| Group I | Control (Normal Saline) | 13.0±0.2673 |
| Group II | Sodium Nitrite | 7.625±0.37 a** |
| Group III | HAE of L.siceraria +Sodium Nitrite | 10.00±0.56 b* |
| Group IV | HAE of L.siceraria +Sodium Nitrite | 9.750±0.453 b** |
| Group V | Alteplase+Sodium Nitrite | 12.25±0.366b** |
Values are given as Mean ±SEM for n=8 in each group, comparison were made between a)Group I and Group II with Group III, Group IV, Group V* symbol statistical significance done by one way ANOVA followed by Dunnett’s test P<0.01.
Adhesion Test
Animals of all groups were subjected to Cylinder test on day 14 of drug treatment. The HAE of L.siceraria (200mg/kg,400mg/kg) treated groups showed significant decrease in time in dose dependent manner whrn compared with disease control group as shown in table III.
Table 3: Effect of HAE siceraria on Adhesion test
| Groups | Treatment | Adhesion test time (sec) |
| Group I | Control (Normal Saline) | 59.375±2.745 |
| Group II | Sodium Nitrite | 142.50±8.763 a** |
| Group III | HAE of L.siceraria +Sodium Nitrite | 100.625±6.156 b* |
| Group IV | HAE of L.siceraria +Sodium Nitrite | 87.50±7.008 b** |
| Group V | Alteplase+Sodium Nitrite | 81.875±6.810b** |
Values are given as Mean ±SEM for n=8 in each group, comparison were made between a)Group I and Group II b) Group II with Group III, Group IV, Group V* symbol statistical significance done by one way ANOVA followed by Dunnett’s test P<0.01.
Effect of Glutathione (GSH)
Neuroprotective effect of HAE of L.siceraria was supported by increased neuronal antioxidant level. Decreased level of GSH were observed in brain tissue of disease control group significant increase in GSH level was observed in HAE of L.siceraria (200mg/kg,400mg/kg) treated groups. This increased in GSH level is possibly required to overcome excessive oxidative stress. As shown in table IV.
Table 4: Effect of HAE siceraria on glutathione levels
| Groups | Treatment | Glutathione µg/mg of Tissue |
| Group I | Control (Normal Saline) | 32.875±0.350 |
| Group II | Sodium Nitrite | 15.0±1.180 a** |
| Group III | HAE of L.siceraria +Sodium Nitrite | 20.375±0.778 b* |
| Group IV | HAE of L.siceraria +Sodium Nitrite | 24.00±0.625 b** |
| Group V | Alteplase+Sodium Nitrite | 31.0±0.756b** |
Values are given as Mean ±SEM for n=8 in each group, comparison were made between a) Group I and Group II b) Group II with Group III, Group IV, Group V* symbol statistical significance done by one way ANOVA followed by Dunnett’s test P<0.01.
Effect of Nitrates
A significant increase in the plasma nitrates was observed in hypoxia induced animals when compared with control group. Whereas administration of HAE of L.siceraria (200mg/kg, 400mg/kg) decreased the nitrates level in comparison to sodium nitrite treated group. As shown in table V.
Table 5: Effect of HAE siceraria on nitrate levels
| Groups | Treatment | Nitrates µg/ml |
| Group I | Control (Normal Saline) | 12.250±0.559 |
| Group II | Sodium Nitrite | 47.875±1.630 a** |
| Group III | HAE of L.siceraria +Sodium Nitrite | 38.625±1.362 b* |
| Group IV | HAE of L.siceraria +Sodium Nitrite | 22.875±1.246 b** |
| Group V | Alteplase+Sodium Nitrite | 14.500±0.567b** |
Values are given as Mean ±SEM for n=8 in each group, comparison were made between a) Group I and Group II b) Group II with Group III, Group IV, Group V* symbol statistical significance done by one way ANOVA followed by Dunnett’s test P<0.01.
Effect of TBAR’s
A significant increase in TBAR’s level in brain was observed in hypoxia induced animals when compared with control group. Whereas administration of HAE of L.siceraria (200mg/kg,400mg/kg) decreased the TBAR’s level in comparison to sodium nitrite treated groups. As shown in table VI
Table 6: Effect of HAE siceraria on tbar’s levels
| Groups | Treatment | TBAR’s µM/mg |
| Group I | Control (Normal Saline) | 0.157±0.004 |
| Group II | Sodium Nitrite | 0.359±0.006 a** |
| Group III | HAE of L.siceraria +Sodium Nitrite | 0.322±0.005 b* |
| Group IV | HAE of L.siceraria +Sodium Nitrite | 0.306±0.006 b** |
| Group V | Alteplase+Sodium Nitrite | 0.166±0.005b** |
Values are given as Mean ±SEM for n=8 in each group, comparison were made between a) Group I and Group II b) Group II with Group III, Group IV, Group V* symbol statistical significance done by one way ANOVA followed by Dunnett’s test P<0.01.
Effect of Dopamine
A significant decrease in dopamine level in brain was observed in hypoxia induced animals when compared with control group. Whereas administration of HAE of L.siceraria (200mg/kg,400mg/kg) increased the dopamine level in comparison to sodium nitrite treated group. As shown in table VII.
Table 7: Effect of HAE siceraria on dopamine levels
| Groups | Treatment | Dopamine µg/mg of Tissue |
| Group I | Control (Normal Saline) | 64.3±14.56 |
| Group II | Sodium Nitrite | 40.1±10.6 a** |
| Group III | HAE of L.siceraria +Sodium Nitrite | 43.0±11.92 b* |
| Group IV | HAE of L.siceraria +Sodium Nitrite | 51.2±12.24 b** |
| Group V | Alteplase+Sodium Nitrite | 61.0±0.756b** |
Values are given as Mean ±SEM for n=8 in each group, comparison were made between a) Group I and Group II b) Group II with Group III, Group IV, Group V* symbol statistical significance done by one way ANOVA followed by Dunnett’s test P<0.01.
Histopathological study
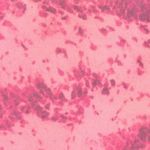 |
Figure 1: Group I (full neurons, nuclei tightly arranged are light shaded)
|
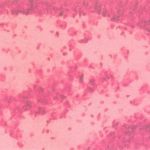 |
Figure 2: Group II (shrunken neurons cytoplasm, nuclei moved side and dark stained)
|
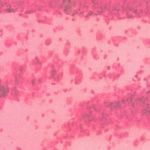 |
Figure 3: Group III (Nuclei lightly stained and neurons arranged tightly)
|
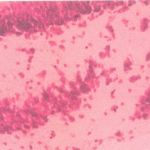 |
Figure 4: Group IV (Nuclei lightly stained and neurons arranged tightly)
|
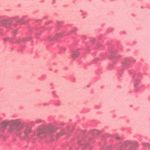 |
Figure 5: Group V (Full neurons, tightly arranged nuclei are light shaded)
|
Conclusion
The level of Dopamine and Glutathione was significantly reduced in disease control when compared with normal group, upon treatment it increased the level in dose dependant manner and the level of Nitrates and TBAR’s significantly increased in negative control when compared with that of normal control group, upon treatment it decreased the level in dose dependant manner.
The present study thus concludes that L.siceraria may be effective in the theraphy of various neurodegenerative diseases, which may be due to effective free radical scavanging property of the plant.
References
- Kobayashi T, Mori Y, “ca2+ channel antagonists and neuroprotection from cerebral ischemia”. Eur J Pharmacol. 1998;363:1-15.
- Astrup J, Siesjo BK, Symmon L. ” Theresholds in cerebral ischemmia-theischemic penumbra. Stroke. 1981;15:723-725.
- Hossmann KA. “Viability theresholds and the penumbra of focal ischemia”. Ann Neurology. 1994;36:557-565.
- Y.K. Gupta et al. “Animal model for cerebral ischemia for evaluation of drugs”. Indian J Physiol Pharmacol.2004;48(4):379-394.
- Xuejiang W, Magara T, Konishi T. “Prevention and repair of cerebral ishcemia-reperfusion injury by chinese herbal medicine, shengmai san, in rats”. Free Radic Res. 1999;31(5):449-455.
- Singh M.K et al. “Protective effect of langenaria siceraria against doxorubicin induced cardiotoxicity in wistar rats”. Int J. Drug Dev & Res. April-June 2012:4(2):298-305.
- Evans WC. Trees and Evans Pharmacognosy. London Balliere Tindall Publishers 1996. 99.388-438.
- Premanand R et al “Neuropotecctive efffects of Abrus precatorius Linn. aerial extract on hypoxic neurotoxicity induced rats”. IJCPS, 2010. Aug. Vol.1(1).
- Michelle J. Porritt et al. Photothrombosis-induced Infarction of the Mouse Cerebral Cortex is not affected by the Nrf2-activator”. Plos One,2012, INssue 7,vol.7. pp.1090-1096.
- Subramaniyam navaneetha krishna et al. ” Free radical scavenging activity of Evolvuhes alsionoldes on Hypoxia induced Nuerodegeneration. IJRSP. 2011;2(1), 302-305.
- P Muralidharan ete al. “Cerebroprotective effect of Glycyrrhiizza glabra Linn. root extract onn hypoxic rats. “Bangladesh J Pharmmacol. 2009;4:60-64.
- Green L.c, Wagner,et al. “Analysis of Nitrate in Biological fluid. Analytical Bio chem. 1982;131-136.
- Dongren Yang et al. “Polychlorinated biphenyls increases apoptosis in the developing. IND Medica current Neuro Biology;1(1).
- J. Hofmeijer eti al.”Delayed Decompressive surgery Increase Apparent Diffusion Coeffficient and Improves Peri-Infarct Perfusioyingn in Rats with Space-Occupying cerebral infarction”, Stroke. 2004:35:1476-1481.








