Manuscript accepted on :February 10, 2016
Published online on: --
Plagiarism Check: Yes
Mohammed Said Nedjimi and Lakhdar Sekhri*
Laboratoire de Dynamique Interaction et Réactivité des Systèmes, Process Engineering Department, Faculty of Applied Sciences, University Kasdi Merbah, Ouargla 30000, Algeria. Corresponding Author E-mail: sekhril@yahoo.fr
DOI : https://dx.doi.org/10.13005/bpj/960
Abstract
The present work is aimed mainly to investigate and find novel route for the most important, fundamental and practical asymmetric reduction reaction of prochiral ketones in order to produce non-racemic chiral alcohols, from which many industrially important chemicals such as pharmaceuticals, agrochemicals, and natural products. This study underscored the bioreduction of various acetophenones: Acetophenone, 4'-nitroacetophenone and 4'-haloacetophenones (X=F, Cl, and Br) were chosen as the model substrates for simple ketones, nitro and halogen-containing aromatic ketones. Cynara scolumus L., Terfezia sp and Phoenix dactylifer L. were chosen as the biocatalysts. It was found that these kinds of prochiral ketones could be reduced by these plants tissue with high enantioselectivity. Both R- and S-form configuration chiral alcohols could be obtained. The ee and chemical yield could reach about 89.0 and 77.2% respectively for acetophenone, 82.0 and 52.0% respectively for 4'-nitroacetophenone and 96.5 and 65.5% respectively for 4'-haloacetophenones (X=F, Cl, and Br) reduction reaction with favorable plant tissue. The results obtained in the present study suggest that the Cynara Scolumus L, Terfezia sp and Phoenix dactylifera L can be used as biocatalysts in synthesis many enantiomerically pure pharmaceuticals.
Keywords
Biocatalysts; chiral alcohols; acetophenones; Phoenix dactylifera; Cynara Scolumus
Download this article as:| Copy the following to cite this article: Nedjimi M. S, Sekhri L. Asymmetric Bioreduction of Prochiral Ketones Catalyzed by Cynara Scolumus L, Terfezia Sp and Phoenix Dactylifera L. Biomed Pharmacol J 2016;9(2). |
| Copy the following to cite this URL: Nedjimi M. S, Sekhri L. Asymmetric Bioreduction of Prochiral Ketones Catalyzed by Cynara Scolumus L, Terfezia Sp and Phoenix Dactylifera L. Biomed Pharmacol J 2016;9(2). Available from: http://biomedpharmajournal.org/?p=7798 |
Introduction
In recent years, chemicals reactions using plant cell cultures and part of plants as biocatalysts have received great attention.1-3 This crescent interest is due to the wide biotechnological potential of the enzymatic reactions. The biocatalytic transformations using plants can be applied in bioreduction of ketones,4-6 enzymatic lactonization,7 hydrolysis of esters,8 addition of hydrogen cyanide,9 and hydroxylation and oxidation reaction.10 The asymmetric reduction of Prochiral ketones is one of the most important, fundamental and practical reaction for producing non-racemic chiral alcohols, from which many industrially important chemicals such as pharmaceuticals, agrochemicals, and natural products.
The catalysts for the asymmetric reduction of prochiral ketones can be classified into two categories: chemical and biological methodologies. Presently, there are five chemical reagents which are extensively used in asymmetric reduction: Brown’s DIP chloride,11,12 Midlands Alpin-Boranes,13 Corey’s oxazaborolidines,14,15 Nyori’s BINAL-H and BINAP-Ru complexes.16 In 2000, L. Sekhri and N. J. Lawrence utilized Corey’s oxazaborolidine to obtain excellent yields and enantioselectivities for a variety of diphenylphosphynoyl alcohols17. The biological catalysts used for the asymmetric reductions, baker’s yeast, 18-21 and vegetables,22 germinated plant23 has been applied to organic synthesis because these biocatalysts are easily obtainable from markets and easily manipulated. An increasing number of reports dealing with the assessment of bioreduction of prochiral ketones using plants are frequently available.24-30
Following with our studies in asymmetric reduction of prochiral ketones, either using chemical17 or biological27 methodologies, we wish to report this study in order to explore the asymmetric reduction of different kinds of prochiral ketones catalyzed by various plants tissue. Acetophenone 1a was chosen as a preferred model substrate of simple ketones; 4-chloroacetophenone 2a was chosen as the model substrate of halogen-containing aromatic ketones such as 4′-bromoacetophenone 3a and 4′-fluoroacetophenone 4a, since the single enantiomer of halogen-containing aromatic alcohols is one of the most important kinds of kiral building blocks for many enantiomerically pure pharmaceuticals, such as L-chlorprenaline, R-tomoxetine, S-fluoxetine, R-salbutamol, and R-denopamine.31 Cynara Scolumus L, Terfezia sp and Phoenix dactylifera L were chosen as the biocatalyst, since the literature concerning these plants contains little or no information on its uses as biocatalysts.
Cynara scolumus L., Terfezia sp and Phoenix dactylifer L. are known locally as “Karnoun”, “Terfas or Elkamaa” and “Djmar” respectively.
Experimental
General Methods
Acetophenone 1a, 4′-chloroacetophenone 2a, 4′-bromooroacetophenone 3a, 4′-fluoroacetophenone 4a and 4′-nitroacetophenone 5a, were purchased from Aldrich. These chemicals were used without further purification. Thin-chromatography (TLC) was performed using precoated plates (Aluminium foil, silica gel 60 F254 Merck, 0.25mm). Merck 60 silica gel (230-400 mesh) was used for flash chromatography. Optical rotations were determined on Euromex Polarimeter PM. 5400 (Mitscherlich type polarimeter).
All 300 MHz and 75 MHz 13C NMR spectra were run on a Bruker AC 300 NMR spectrometer. Both 1H NMR and 1C NMR spectra were recorded using CHCl3 as internal standard; Infrared spectra were recorded using a Perkin-Elmer 783 spectrometer equipped with a PE 600 data station
Biocatalysts
Fresh Cynara scolumus L., Terfezia sp and Phoenix dactylifer L. were obtained from a local market. Terfezia sp grows in the disert (south of Algeria): Laghouat, El-Beith, Ghardia and Ouargla; Phoenix dactylifer L. was taken from the palm pulp stalks. To increase the contact of the substrate with the biocatalyst, the external pulp of the plants was removed and the rest was carefully cut into small thin pieces (approximately 1 cm long slice).
Synthesis of the chiral alcohols 1b-5b
The chiral alcohols 1b-5b were synthesized by reduction of the corresponding acetophenones 1a-5a using Cynara Scolumus L, Terfezia sp and Phoenix dactylifera L as biocatalysts.
General procedures for biotransformations
Typical reaction mixture of (0.02 mol) appropriate ketones 1a-5a, 2% (W/V) of glucose or i-PrOH (in the case of solid ketones), 20 ml of phosphate buffer (pH = 6.5) was added to 25g of cultured plants suspension in 80 mL deionized water. The reaction mixture was agitated in orbital incubator shaker (150 rpm) at 30°C for the time indicated in the tables 1-4. The progress of the reaction was monitored by TLC. The plants pieces were then removed by filtration, washed with deionized water and the filtrate was extracted with petroleum ether (3x100ml). The petroleum ether fraction was dried over anhydrous (MgSO4) and the solvent was evaporated to get the final product. Then chemical yield and enantio selectivity were determined. Each experiment was parallelly repeated at least three times. Then the average value and standard deviations were given.
The products were identified by comparing their data with those of authentic samples on TLC, by IR, and 1HNMR spectra. The presence of alcoholic group in the final product was chemically confirmed by acetyl chloride test.
Identification of chiral alcohols 1b-5b by optical properties spectroscopic data
Phenylethanol (1b) Using Cynara scolumus L
(R)-(1b) was obtained in 30% yield, +24 (c 5, MeOH). The absolute configuration (R)-(1b) was estimated by analogy with {Lit.,32 +45 (c 5, MeOH) for R-isomer}; Using Terfezia sp: (R)-(1b) was obtained in 21.4% yield, +10 (c 5, MeOH). The absolute configuration (R)-(1b) was estimated by analogy with {Lit.,32 +45 (c 5, MeOH) for R-isomer}; Using phoenix dactylifera L: (R)-(1b) was obtained in 68.6% yield, +40 (c 5, MeOH). The absolute configuration (R)-(1b) was estimated by analogy with {Lit.,32 +45 (c 5, MeOH) for R-isomer}. The IR and 1H and 13C NMR spectra of (1b) were identical to those of authentic samples.33,34 1H (CDCl3, 300 MHz): δ (ppm): 1.5 (3H, d, CH3CHOH-), 4.7 (1H, br.s, OH), 5.2 (1H, q, –CHOH), 7.3-7.4 (4H, m, Ar-H); 13C (CDCl3, 75 MHz): δ (ppm): 22.8 (CH3CHOH), 69.9 (-CHOH), ), 127.1 (-CH, Ar), 127.6 (-CH, Ar), 128.9 (-CH, Ar), 146.1 (C, Ar); νmax (KBr Disk, Cm-1): 3340-3060 (OH).
4′-Chlorophenylethanol (2b) Using Cynara scolumus L
(S)-(2b) was obtained in 29.45% yield, -35.7 (c 5, EtOH). The absolute configuration (S)-(2b) was estimated by analogy with {Lit.,35 -37 (c 5, EtOH) for S-isomer}; Using Terfezia sp: (S)-(2b) was obtained in 37% yield, +17.4 (c 5, EtOH). The absolute configuration (R)-(2b) was estimated by analogy with {Lit.,35 +37 (c 5, EtOH) for R-isomer}; Using phoenix dactylifera L: (R)-(2b) was obtained in 51.5% yield, +28.5 (c 5, EtOH). The absolute configuration (R)-(2b) was estimated by analogy with {Lit.,35 +37 (c 5, EtOH) for R-isomer}. The IR and 1H and 13C NMR spectra of (2b) were identical to those of authentic samples.33,34 (1H (CDCl3, 300 MHz): δ (ppm): 1.3 (3H, d, CH3CHOH-), 3.5 (1H, br.s, OH), 4.7 (1H, q, –CHOH), 7.0-7.3 (4H, m, Ar-H); 13C (CDCl3, 75 MHz): δ (ppm): 28.08 (CH3CHOH), 69.54 (-CHOH), ), 126.93 (-CH, Ar), 128.25 (-CH, Ar), 132.94 (C, Ar), 144.44 (C, Ar); νmax (KBr Disk, Cm-1): 3340-3060 (OH).
4′-Bromophenylethanol (3b) Using Cynara scolumus L
(S)-(3b) was obtained in 42.7% yield, -31 (c 5, CHCl3). The absolute configuration (S)-(3b) was estimated by analogy with {Lit.,35 -39 (c 5, CHCl3) for S-isomer}; Using Terfezia sp: (S)-(3b) was obtained in 33% yield, +11 (c 5, CHCl3). The absolute configuration (R)-(3b) was estimated by analogy with {Lit.,35 +39 (c 5, CHCl3) for R-isomer}; Using phoenix dactylifera L: (R)-(3b) was obtained in 59.0% yield, +30 (c 5, CHCl3). The absolute configuration (R)-(3b) was estimated by analogy with {Lit.,35 +39 (c 5, CHCl3) for R-isomer}. The IR and 1H and 13C NMR spectra of (3b) were identical to those of authentic samples.33,34 1H (CDCl3, 300 MHz): δ (ppm): 1.5 (3H, d, CH3CHOH-), 4.7 (1H, q, –CHOH), 5.2 (1H, br.s, OH), 7.3-7.9 (4H, m, Ar-H); 13C (CDCl3, 75 MHz): δ (ppm): 28.03 (CH3CHOH), 69.54 (-CHOH), ), 126.93 (-CH, Ar), 128.25 (-CH, Ar), 132.94 (C, Ar), 144.44 (C, Ar); νmax (KBr Disk, Cm-1): 3340-3060 (OH).
4′-Fluorophenylethanol (4b). Using Cynara scolumus L
(S)-(4b) was obtained in 20% yield, -35 (c 5, CHCl3). The absolute configuration (S)-(4b) was estimated by analogy with {Lit.,32 -50 (c 5, CHCl3) for S-isomer}; Using Terfezia sp: (S)-(4b) was obtained in 16% yield, +25 (c 5, CHCl3). The absolute configuration (R)-(4b) was estimated by analogy with {Lit.,32 +50 (c 5, CHCl3) for R-isomer}; Using phoenix dactylifera L: (R)-(4b) was obtained in 55.5% yield, +40 (c 5, CHCl3). The absolute configuration (R)-(4b) was estimated by analogy with {Lit.,32 +50 (c 5, CHCl3) for R-isomer}. The IR and 1H and 13C NMR spectra of (4b) were identical to those of authentic samples.50-51 1H (CDCl3, 300 MHz): δ (ppm): 1.4 (3H, d, CH3CHOH-), 3.2 (1H, br.s, OH), 4.8 (1H, q, –CHOH), 6.8-7.0 (2H, m, Ar-H), 7.1-7.3 (2H, m, Ar-H); 13C (CDCl3, 75 MHz): δ (ppm): 22.8 (CH3CHOH), 69.9 (-CHOH), ), 115.7 (-CH, Ar), 126.9 (-CH, Ar), 141.7 (C, Ar), 161.8 (C, Ar); νmax (KBr Disk, Cm-1): 3340-3060 (OH).
4′-Nitrophenylethanol (5b) Using phoenix dactylifera L
(5b) was obtained in 21.5% yield; Using phoenix dactylifera L: (R)-(5b) was obtained in 52% yield, +30 (c 5, MeOH). The absolute configuration (R)- (5b) was estimated by analogy with {Lit.,36 +31(c 5, MeOH) for R-isomer}. The IR and 1H and 13C NMR spectra of (5b) were identical to those of authentic samples.33,34 1H (CDCl3, 300 MHz): δ (ppm): 1.4 (3H, d, CH3CHOH-), 2.6 (1H, br.s, OH), 4.9 (1H, q, –CHOH), 7.4 (2H, d, Ar-H), 8.1 (2H, d, Ar-H); 13C (CDCl3, 75 MHz): δ (ppm): 25.27 (CH3), 69.27 (-CHOH), ), 123.56 (-CH, Ar), 126.04 (-CH, Ar), 146.89 (C, Ar), 153.23 (C, Ar); νmax (KBr Disk, Cm-1): 3340-3060 (OH).
Results and Discussion
Asymmetric transformations invariably involve the conversion of two dimentional substrate into a three dimentional product. For prochiral ketones such as acetophenone reduction shown in scheme-1, addition to the back face gives 1-phenyl alcohol with R configuration, while addition to the back face gives alcohol with S configuration.The problem of course is that most common reducing agents, such as sodium borohydride or lithium aluminium hydride, react equally readily with either face. The most obvious solution to this problem is to use a hydride source which itself is enantiomerically pure in principal such as reagent will transfer the hydride to each face of the ketone through diastereoisomerically distinct transition state, which gives at least a fighting chance of an enrgy difference, and preference for addition to one face over the other.
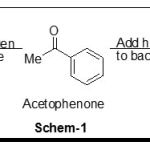 |
Scheme 1
|
Moreover, plants are potential biocatalysts used as the alternative solution to this problem, since they are easily obtainable from markets and easily manipulated. Asymmetric reduction reactions of acetophenone 1a, 4-chloroacetophenone 2a, 4′-bromoacetophenone 3a, 4′-fluoroacetophenone 4a and 4′-nitroacetophenone 5a by various plants tissue was investigated (Scheme-2).
Cynara scolumus L., Terfezia sp and Phoenix dactylifer L. was selected as the biocatalysts.
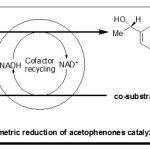 |
Scheme 2
|
Bioreduction of various acetophenones with Cynara scolumus L.
Acetophenone is a preferred model substrate of simple ketones and aromatic ketones for asymmetric reaction. Acetophenone could be reduced to chiral 1-phenylethanol with attractive enantioselectivity. The yield and ee are 30 and 53% respectively catalyzed by Cynara scolumus L. and the reaction reached the equilibrium within 2 days (48h). Moreover, the best results can be obtained by asymmetric reduction of 4′-haloacetophenones (X=F, Cl, and Br) and 4′-ntroacetophenone by Cynara scolumus L. in terms of the yield and ee. The yield and ee are (29-36) and (71.4-96.5) % and the reaction reached the equilibrium within 4 days (96 hours). The halogen-containing aromatic ketones such as 4′-chloroacetophenone is more acceptable to plant cells than simple aromatic ketone and the halogen bond on the phenyl group enlarges the difference between the two groups on both sides of the carbonyl, therefore improving the enantioselectivity of this reduction reaction. The results of asymmetric reduction of various acetophenones by Cynara scolumus L. are summarized in table-1.
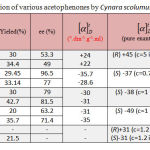 |
Table 1: Bioreduction of various acetophenones by Cynara scolumus L
|
Bioreduction of various acetophenones with Terfezia sp
The halogen-containing aromatic alcohols can be synthesized by asymmetric reduction of the corresponding prochiral halogen containing aromatic ketones. To investigate the asymmetric reduction of the halogen containing aromatic ketones catalyzed by Terfezia sp, 4′-chloroacetophenone was chosen as the model substrate, since it posses the general peculiarities of this kind of ketones. Moreover, the products, R-or S-1-(4′-chlorophenyl) ethanol, are key chiral intermediates for many chiral drugs.31 The results were shown in table 2.
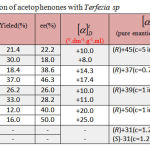 |
Table 2: Bioreduction of acetophenones with Terfezia sp
|
It was observed that the reaction results of asymmetric reduction of 4′-chloroacetophenone 2a, 4′-bromoacetophenone 3a and 4′-fluoroacetophenone 4a by this plant tissue are similar to that of acetophenone. Even more interesting is that the enantioselectively is (28-50) % to this plant, remarkably higher than that of acetophenone reduction reaction. This indicates that the halogen-containing aromatic ketone is more acceptable to plant cells than simple aromatic ketone and the halogen bond on the phenyl group enlarges the difference between the two groups on both sides of the carbonyl, therefore improving the enantioselectivity of this reduction reaction.
Bioreduction of various acetophenones with Phoenix dactylifera L
The product chemical yield and enantioselectivity of the reduction reaction are shown in Table 3. The best results were obtained from the plant, Phoenix dactylifera L, as a biocatalyst. The yield and ee are excellent (51.5-77.2) % and (66-89) % respectively compared with those obtained with tested plants, Cynara scolumus L.and Terfezia sp.
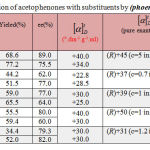 |
Table 3: Bioreduction of acetophenones with substituents by (phoenix dactylifera L
|
Determination of optical activity of chiral products
Optical properties of the products obtained from the prochiral were studied with the help of polarimeter using the following method. c% solution of the chiral alcohols in a suitable solvent (methanol or chloroform) was prepared and introduced to the polarimeter tube. The optical rotation values were determined individually for the product of each of the prochiral substrates. Specific rotation values are then calculated using the relation (eq. 1).
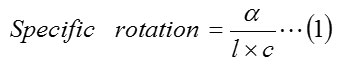
Where,
α = angle of rotation
l = length of polarimeter tube (1dm)
c = concentration of the solution (g/ml)
Further enantiomeric excess values of the chiral products are determined by the equation (eq. 2)
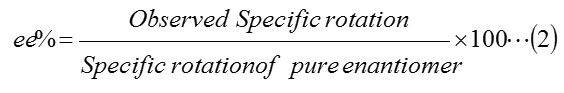
Conclusion
This study underscored the bioreduction of various acetophenones with Cynara scolumus L., Terfezia sp and Phoenix dactylifer L. Acetophenone, 4′-haloacetophenones (X=F, Cl, and Br) and 4′-ntroacetophenone can be effectively reduced to the corresponding chiral alcohols by the applied plants tisse. Moreover, both R- and S- form configuration chiral alcohols could be obtained through these asymmetric reduction reactions. Acetophenone could be reduced to chiral 1-phenylethanol with attractive enantioselectivity. The yield and ee are 30 and 53.3% respectively catalyzed by the tested plant: Cynara scolumus L. and the reaction reached the equilibrium within 2 days (48h). However, the results of asymmetric reduction of 4′-haloacetophenones (X=F, Cl, and Br) and 4′-nitroacetophenone by Cynara scolumus L. are more attractive in terms of the yield and ee. The yield and ee are (29-36) and (71.4-96.5) % respectively and the reaction reached the equilibrium within 2-4 days (48-96 hours). The reason is that 4′-nitroacetophenone and halogen-containing aromatic ketones such as 4′-chloroacetophenone is more acceptable to plant cells than simple aromatic ketone. The favorable plant tissue to all tested acetophenones is Phoenix dactylifera L based on the chemical yield (52.0-77.2%) and ee results (60.0-89.0%). However, with the plant tissue, Terfezia sp , the ee and yield were not so satisfactory as shown table 2 for the reduction of all tested acetophenones.
This provides a new route to produce chiral alcohols, as the platform chemicals for enantiomerically pure pharmaceuticals, through asymmetric reduction of the corresponding prochiral ketones. The synthetic chiral alcohol drugs are useful in the treatment of several health problems; bacterial infections such as the urinary tract infection, that is the most common bacterial diseases in children, as it ranks second in terms of spreading infection after respiratory tract.
Therefore, we have chosen the study of asymmetric bioreduction of prochiral ketones catalyzed by Cynara Scolumus L, Terfezia sp and Phoenix dactylifera L in order to synthesise chiral alcohols, because they are useful in the treatment of several health problems and has several significant applications.
Acknowledgment
This work was supported by the Algerian Ministry of Higher Education and Scientific Research (MHESR).
References
- Giri, A., Dhinga, V., Giri, C. C., Singh, A., Ward, O. P., Narsu, M. L. Biotechnol. Adv., 2001; (19): 175-185.
- Villa, R., Molinari, F., Levati, M., Aragozzini, F. Biotechnol. Lett., 1998 (20):1105.
- Bruni, R., Fantin, G., Medici, A., Pedrini, P., Sacchetti, G. Tetrahedron Lett., 2002 (43): 3377-3385.
- Machado, L. L., Souza, J. S. N., Mattos, M. C., Sakata, S. K., Cordell, G. A., Lemos, T. L. G. Phytochemistry, 2006; (67): 1637-1643.
- Rodriguez, P., Barton, M., Aldabalde, V., Onetto, S., Panizza, P., Menendez, P., Gonzalez, D., Rodriguez, S., J. Mol. Catal. Enzyme, 2007; (49): 8-11.
- Orden, A. A., Bisogno, F. R., Giordano, O. S., Sanz, M. K., J. Mol. Catal. Enzyme, 2008; (51): 49-55.
- Olejniczak, T., Mironowicz, A., Wawwrzenczyk, C., Bioorg., Chem., 2003; (31): 109.
- Maczka, W. K., Mironowicz, A., Tetrahedron: Asymm., 2002; (13): 2299.
- Hamandez, L., Luna, H., Ruiz-Teran, F., Vasquez, A., J. Mol. Catal. B: Enzyme, (30) 2004; 105.
- Sakamaki, H., Itoh, K., Taniai, T., Kitanaka,,Takgi, Y., Chai, W., Horiuchi, C. A., J. Mol. Catal. B: Enzyme, 2005; (32): 103.
- Brown, H. C., Park, W. S., Cho, B. T., Ramachandran, P. V., Org. Chem., 1987; (52): 5406
- Coppola, G. M., Schuster, H. F., Asymmetric Synthesis, Construction of chorale molecules using aminoacids, Wiley-interesc, Publ., Chapter1, 2 and 3 (1987).
- Midland, M. M., Rev., 1989; (89): 1553
- Corey, E. J., Bakshi, R. K., Shibata, S., J. Am. Chem. Soc., 1987; (109): 5553.
- Corey, E. J., Bakshi, R. K., Shibata, S., Singh, V. K., Am. Chem. Soc., 1987; (109): 7925.
- Henry, B. K., Maurice, T., Jean, C. F., Tetrahedron Lett., 1991; (42): 4959
- Sekhri, L., Lawrence, N. J., J. Soc. Alger. Chim., 2000; 1 (10): 9-21
- Yasohara, Y., Kizaki, N., Hasegawa, J., Takahashi, S. Wada, M., Katoka, M., Shimizu, S., Appl. Microbial. Biotechnol., 1999; (51): 847
- Wada, M., Katoka, M., Kawabata, H., Yasohara, Y., Kizaki, N., Hasegawa, J., Takahashi, S., Shimizu, S., BioSc. Biotech. Biochem., 1999; (62): 280.
- Ferabochi, P., Grisenti, P., Manzocchi, A., Santaniello, E., J. Chem. Soc., Perkin, 1990; (1): 2469-2480
- Ramaswamy, S., Oehischlager, A. C., Tetrahedron, 1991; (47): 1145-1152.
- Utsukihara, T., Watanabe, S., Tomiyama, A., Chai, W., Horiuchi, C. A., J. Mol. Catal. Enzyme, 2006; (41): 103-109
- Matsuo, K., Kawabe, S., Tokuda, Y., Eguchi, T., Yamanaka, R., Nakumera, K., Tetrahedron: Asummetry, 2008; (19): 157-168
- Akakabe, Y., Takahashi, M., Kamezawa, M., Kikuchi, K., Tachibana, H., Ohttani, T., Naoshima, Y., J. CHEM. SOC. PERKIN TRANS. 1295-207.
- Phukan, K., Devi, N., International Journal of ChemTech Reasearch, 2012; 4(1): 203-207.
- Matsuo, K., Kawabe, S., Tokuda, Y., Eguchi, T., Yamanaka, R. Nakumera, K., Tetrahedron: Asymmetry, 2008; (19): 157-159.
- Sekhri, M. Nedjimi, Biomedical and Pharmacology Journal, 2009; 2(2): 287-292
- Yang, Z. H., Zeng, R., Yang, G., Wang, Y., Zhen Li, L., Zao-Sheng Lv, Yao, M., Lai, B., J Ind Microbiol Biotechnol, 2008; (35): 1047-1051.
- Andrade, L. H., Utsunomiya, R. S., Omori, A. T., Porto, A. L. M., Comasseto, J. V., Journal of molecular Catalysis B: Enzymatic, 2006; (38): 84-90.
- Selvi, N. A., Chattopadhyay, S., Tetrahedron, 2001; (57): 2833-2839.
- Serbnik M., Ramachandran P. V., Brown, H. C., J. Org Chem., 1988; (53): 2916-2920.
- Aldrich Catalogue Handbook of fine Chemicals, 1995/1996.
- Sekhri, L. PhD thesis, Constantine University, Algeria, 1998.
- Drew, M. D., Fontaine, D., Lawrence, N. J., Fontaine, D., Sekhri, L., Synlett., 1997; 989-991.
- Basavaiah, D., Venkates , K., Sekhara Reddy,, Tetrahedron: Asymmetry, 2006; (17): 1041-1044.
- Anwar, S., Periasamy, M., Tetrahedron: Asymmetry, 2006; (17): 3244-3247.







