Thilip Kumar Gnanadurai1, Hemamalini Ramasamy Vajravelu2, Prabhavathi Krishnan3, Balaji R4, Saravanan Ayyavoo5 and Jamuna Rani R6
1Department of PhysiologySRM Medical College, Hospital and Research Centre.
2Department of Community Medicine SRM Medical College, Hospital and Research Centre.
3Department of Pharmacology SRM Medical College, Hospital and Research Centre.
DOI : https://dx.doi.org/10.13005/bpj/982
Abstract
Cigarette smoking is a worldwide major risk factor for the development of atherosclerosis, coronary heart disease, acute myocardial infarction and sudden cardiac death in the younger age in south Asia. Smoking affects the cardiovascular system by several mechanisms. The present study was planned to study the effect of smoking on sympathetic nerve activity by isometric handgrip test in normal healthy young adults. Hundred male subjects in the age group of 18 to 25 years were involved. It included 50 smokers and 50 non – smokers (control group).Sympathetic nerve activity was assessed by isometric hand grip test. BMI, hand grip strength, endurance time, blood pressure before and after hand grip, and diastolic difference were assessed and compared between both the groups. Statistical analysis was done using SPSS 21. Student t test was performed to compare the variables between two groups.There was statistically significant increase in resting systolic pressure (p<0.01), diastolic Pressure (p<0.01), resting Heart Rate (p<0.01), resting mean arterial pressure (p<0.006), and significant diastolic pressure difference (p<0.001) in smokers when compared to nonsmokers. This reflects defect in sympathetic vasomotor tone.Thus the study concludes that smoking possibly has an effect on baroreflex suppression of sympathetic activation in habitual young smokers. Cessation of smoking is associated with reduced cardiovascular mortality and morbidity.
Keywords
Smoking; isometric handgrip exercises; sympathetic nerve; blood pressure
Download this article as:| Copy the following to cite this article: Gnanadurai T. K, Vajravelu H. R, Krishnan P, Balaji R, Ayyavoo S, Rani R. J. Assessment of Sympathetic Nerve Activity by Isometric Handgrip Test in Young Cigarette Smokers. Biomed Pharmacol J 2016;9(2). |
| Copy the following to cite this URL: Gnanadurai T. K, Vajravelu H. R, Krishnan P, Balaji R, Ayyavoo S, Rani R. J. Assessment of Sympathetic Nerve Activity by Isometric Handgrip Test in Young Cigarette Smokers. Biomed Pharmacol J 2016;9(2). Available from: http://biomedpharmajournal.org/?p=7760 |
Introduction
Cigarette smoking is a worldwide major risk factor for the development of atherosclerosis [1] coronary heart disease [2] acute myocardial infarction [3] and sudden cardiac death [4,5] in the younger age group in south Asia[6]. It is considered to be the leading preventable cause of death in the world [7,8].Cigarette smoke is a mixture of several toxic chemicals, of which nicotine, carbon monoxide, and oxidant chemicals are most commonly implicated in the pathogenesis of cardiovascular disease [9]. Smoking or nicotine infusions have been shown to decrease, to not change, to increase plasma nor-epinephrine levels [10, 11] and to decrease [12]or not change [13, 14] the directly recorded muscle sympathetic nerve activity. The effect of smoking on sympathetic activity is not well understood.
Isometric exercise provides a convenient and easy way to activate the cardiovascular system and define the role of the sympathetic nervous system in the exercise response. Isometric muscle contraction evokes large increase in mean arterial pressure, heart rate and muscle sympathetic nerve activity(MSNA) with a minor rise in central hemodynamics [15-17].The increases in MSNA are thought to result, primarily from activation of the muscle metaboreflex or exercise pressor reflex in the exercising muscle [18-20].This reflex originates in sensory receptors which appear to be sensitive to ischemic metabolites generated during muscular contraction, via small myelinated or unmyelinated (group III or IV) afferent fibers, and elicits cardiovascular and vasomotor reflexes [21-24].
Most of the studies have been done to assess the sympathetic nerve activity among the smokers who were diabetics and hypertensive. Our aim was to assess the sympathetic nerve activity in apparently healthy young habitual smokers.
Materials and Methods
Prior to the study commencing, approval for the research was obtained from the institutional Ethics Committee, SRM Medical college hospital and research center and informed consent was obtained from each participant. This was a cross sectional study conducted in the Department of Physiology, SRM Medical college hospital and research center. A total of 100 male subjects in the age group of 18-25 years were selected for this study. Out of these 50 male subjects were mild smokers and remaining 50 were healthy male volunteers who served as control. All participants were physically active and apparently healthy and not participate in any organized endurance exercise training program. Only mild healthy smokers were included in the study group. Smoking index (SI) was calculated as average no. of cigarettes smoked/day x Duration (in years). SI = 1-100 were considered as mild smokers [25]. Individuals with known metabolic and cardiovascular diseases and those taking medications that alter blood pressure, heart rate and autonomic regulation were excluded.
Anthropometric measurements like height in meters and weight in kilogram was measured and the body mass index was calculated. Waist circumference and hip circumference were measured using stretchable inch tape and waist- hip ratio was determined. Participants were instructed to refrain from food and caffeinated products for 4 hours and from vigorous exercise and alcohol for 24 hours before the tests.
Sustained Handgrip Test
The subject was asked to sit comfortably in a chair with their elbow flexed at 90. The subject was explained about the test and the procedure. The baseline BP was recorded by LIPIKIND digital BP monitor. Then the subject was asked to grip the handgrip dynamometer (Rolex Hand grip dynamometer) using maximal isometric force of the dominant hand .Measurements were taken three times 2 minutes apart. The maximum value of the three readings was taken as the maximal voluntary contraction (MVC) (maximal isometric tension i.e., Tmax). Then a mark was made on the dynamometer at 30% of MVC of the subject and then the subject was asked to maintain the sustained grip on the dynamometer up to the mark with uniform intensity till failure and time was noted (Endurance Time).
After the subject had started the contraction, the BP was measured on the contralateral arm at 1st, 2nd 3rd& 4th minute (or any time just before release of grip if it was less than 4 minutes). One more reading was taken 2 minutes after the release of the grip. Subjects were instructed to avoid Valsalva maneuver during the isometric contraction
The BP response was calculated as Highest DBP during the test – Baseline DBP.
Ranges
≥16 mmHg was taken as Normal.
≥11 -15 mmHg as Borderline
<10 mmHg as Abnormal.
Datas were reported as Mean ± SD. Statistical analysis was performed by IBM SPSS software version 21. Unpaired student t test was used to show the difference between cardiovascular parameter such as systolic and diastolic blood pressure, heart rate, pulse pressure, mean arterial pressure, diastolic difference after sustained grip among the control and mild smokers group. The level of significance was set at p < 0.05.
Results
[Table/Fig-1]
| Non-Smokers
(Mean±SE) |
Mild – Smokers
(Mean±SE) |
T test | p-Value | |
| Age(yrs) | 22.36±0.26 | 22.04±0.24 | 0.909 | 0.365 |
| BMI(kg/m2) | 24.59± 0.56 | 25.29±0.66 | 1.261 | 0.100 |
| Waist –Hip Ratio | 0.88 ± 0.02 | 0.90 ± 0.01 | 1.276 | 0.205 |
| Mid_arm circumference(cm) | 27.62 ± 0.46 | 28.24 ± 0.44 | 1.542 | 0.135 |
| Mid_Forearm circumference(cm) | 25.22 ± 0.28 | 25.20 ± 0.39 | 2.045 | 0.144 |
shows the demographic profile of non-smokers and smokers. All were males within the age group of 18-25 years and were non obese. BMI-Body mass index, SD- standard deviation.*p<0.05- significant.
[Table/Fig-2]
| Non-Smokers
(Mean ± SE) |
Mild – Smokers
(Mean ± SE) |
T test | p-Value | |
| Maximum Right Hand strength(kg) | 56.63±6.93 | 54.73±7.7 | 1.376 | .322 |
| Endurance Time(min) | 2.51 ± 0.12 | 2.21 ± 0.12 | 1.744 | 0.084 |
shows the force of contraction and the length of time the muscle were contracted between nonsmokers and smokers. There was no statistically difference between 2 groups.*p<0.05- significant.
[Table/Fig-3]
| Non-Smokers
(Mean±SE) |
Mild – Smokers
(Mean±SE) |
T test | p-Value | |
| Resting Systolic Pressure(mmHg) | 114.54 ± 0.96 | 122.58 ± 1.40 | 4.728 | 0.0001 |
| Resting Diastolic pressure(mmHg) | 70.46 ± 0.92 | 78.12 ± 1.43 | 4.508 | 0.0001 |
| Resting Heart Rate(beats/min) | 77.20±8.14 | 83.167±14.77 | 4.172 | .001 |
| Resting mean arterial pressure(mmHg) | 70.79 ± 0.92 | 92.94 ± 1.35 | 13.567 | 0.0001 |
shows the comparison of basal physiological cardiovascular parameter between nonsmoker and smokers. A statistical significance was found in blood pressure, heart rate, and mean arterial pressure during rest between smoker and non-smokers.*p<0.05- significant.
 |
Figure
|
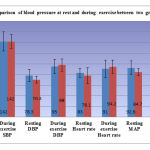
[Table/Fig-4]:
shows the comparison of blood pressure at rest and during exercise between two groups. The mean and standard deviation was depicted for systolic blood pressure (SBP), Diastolic blood pressure (DBP), heart rate (HR) and mean arterial pressure (MAP) at rest and during exercise.
[Table/Fig-5]
| Non-Smokers
(Mean ± SE) |
Mild – Smokers
(Mean ± SE) |
T test | p-Value | |
| Systolic BP difference between rest and during exercise (mmHg) | 28.44 ± 1.75 | 19.48 ± 1.40 | 3.997 | 0.0001 |
| diastolic BP difference between rest and during exercise(mmHg) | 27.52 ± 1.48 | 16.42 ± 0.85 | 6.496 | 0.0001 |
| Herat rate difference between rest and during exercise(mmHg) | 17.4±10.17 | 13.8±10.9 | 4.364 | 0.0001 |
| MAP difference between rest and during exercise(mmHg) | 42.19 ± 1.41 | 17.44 ± 0.78 | 15.356 | 0.0001 |
Comparison of difference between Raise in blood pressure at rest and during exercise.*p<0.05- significant.
[Table/Fig-6]
| Non-Smokers
(Mean ± SE) |
Mild – Smokers
(Mean ± SE) |
T test | p-Value | |
| Diastolic difference (difference between the highest diastolic BP during the test and resting diastolic BP ) | 30.72 ± 1.34 | 16.10 ± 0.82 | 9.300 | 0.0001 |
shows the comparison of Diastolic difference between nonsmoker and smokers. A statistical difference was found between the 2 groups.*p<0.05- significant
[Table/Fig-7]
| Smokers N(%) | Non Smokers N(%) | |
| After 2 Minutes | 6(12) | 50(100) |
| After 5 Minutes | 26(52) | 0 |
| After 10 Minutes | 18(36) | 0 |
shows the comparison of blood pressure after exercise between the two groups. When compared to smokers the healthy nonsmoker’s blood pressure returned to near normal within 2 min whereas smokers returned to their near resting blood pressure in 5-10minutes.
[Table/Fig-8]
| Non-Smokers
(Mean ± SE) |
Mild – Smokers
(Mean ± SE) |
T test | p-Value | |
| Resting Rate Pressure Product | 8751.74 ± 137.19 | 9730.84 ± 309.87 | 2.889 | 0.005 |
| During exercise | 13487.64 ± 382.06 | 13312.24 ± 377.58 | 0.327 | 0.745 |
| After 2 min Rate Pressure Product | 9465.22 ± 272.79 | 9924.58 ± 389.91 | 0.965 | 0.337 |
shows the comparison of Rate Pressure Product at Rest, during exercise and 2 minutes after exercise between nonsmoker and smokers, there was a statistical difference between 2 group at rest but no statistical difference during exercise and after 2 minutes between the 2 groups.*p<0.05- significant.
Discussion
Smoking is one of the important lifestyle risk factor associated with increase morbidity and mortality for cardiovascular disease. A group of researchers observed that nicotine deregulates cardiac autonomic function, boosts sympathetic activation, raises heart rate, causes coronary and peripheral vasoconstriction, increases myocardial workload, and stimulates adrenal and neuronal catecholamine release [26] which established coronary artery diseases.
Numerous factors like age, BMI, waist hip ratio, force of contraction [27] and the length of time the muscle is contracted (Endurance time) [28]influence the sympathetic and pressure response that increase the arterial blood pressure and heart rate .In the present study, there was no significant difference in age, body mass index, waist – hip ratio, maximum grip strength and endurance time in the smokers and non-smokers. So in our study the above factor doesn’t influence the sympathetic activity.
There was a significant increase in resting systolic pressure, resting diastolic pressure, heart Rate and mean arterial pressure in smokers when compared to non-smokers. According to Holly R. Middlekauff [29] the effects of tobacco smoke on sympathetic nerve activity (SNA) depend on the net balance of the sympathetic excitatory effect of cigarette smoke on central neural outflow, and the sympathetic inhibitory effect of the baroreflex, activated by the increase in blood pressure from smoking. When the baroreflex is intact, SNA is suppressed, but when the baroreflex is chronically impaired, SNA increases.The prolonged exposure of nicotine and fine particulate matter in tobacco smoke leads to increased sympathetic nerve activity, which becomes persistent via a positive feedback loop between sympathetic nerve activity and reactive oxidative species. Regarding the baroreflex role on the sympathetic nerve activity two hypotheses has been postulated, one hypothesis states that the reduction in baroreflex sensitivity which increases the resting blood pressure, another hypotheses state that the baroreflex reset takes place.
Thus in our study the resting blood pressure, heart rate and mean arterial pressure increased among smokers which are in accordance with the previous study [30]. Resting heart rate reflects the balance of parasympathetic and sympathetic influences at the sinoatrial node. It assess both parasympathetic and sympathetic activity because of dual innervations of the heart [31, 32]. During isometric exercise the systolic, diastolic blood pressure, heart rate, mean arterial pressure decreased in smokers when compared to non-smokers. On an average the increase in systolic, diastolic blood pressure, heart rate, mean arterial pressure from resting level was to a lesser extent in smokers when compared non-smokers during exercise. . This shows the impairment of sympathetic nerve activity among smokers.
Smokers showed significant decrease in diastolic difference (difference between the highest diastolic BP during the test and Resting Diastolic BP) when compared to non-smokers. This indicates that there was a defect in sympathetic vasomotor tone thus suggesting borderline impairment of sympathetic activity [33]. Nicotine is the principle mediator of chronic effects of smoking on neuro-cardiovascular regulation. These effects are mediated through central nervous system as well as autonomic nervous system [34]. Nonsmoker’s blood pressure returned back to near basal level within 2 minutes where as 20% of the smokers returned to basal level within 2 min, 46.6% of them within 5 minutes, and 33.4% within 10 minutes. This indicates that there is baroreceptor insensitivity among smokers. Rate pressure product is the product of heart rate and systolic blood pressure which indicate the myocardial oxygen utilization. The smokers had a higher rate-pressure product at rest due to their higher resting heart rate when compared to non-smoker. But during static exercise, due to impairment of sympathetic nerve activity, the rate pressure product was lower in smoker when compared to non-smoker. After 2 minute of rest the rate pressure product reached the resting level where as in smokers the rate pressure product did not come back to their resting normal rate. This shows that young mild smokers have an increased workload on the heart, which affects their cardiac performance [35].
Conclusion
Thus the study concludes that there is borderline sympathetic impairment among young mild smokers. This suggests there is baroreflex insensitivity resulting in sympathetic impairment in habitual smokers. Previous studies have showed that baroreflex sensitivity and autonomic function may be restored after smoking cessation .Thus the study would convince young mild smokers to quit smoking.
Reference
- Auerbach 0, Hammond EC, Garfinkel L. Smoking in relation to atherosclerosis of the coronary arteries. N Engl J Med. 1965;273: 775-779.
- Doll R, Hill AB. Mortality of British doctors in relation to smoking: observations on coronary thrombosis. Natl Cancer Inst Monograph. 1966;19:205-215.
- Kahn HA. The Dorn study of smoking and mortality among U.S. veterans: report on eight and one-half years of observation. Nati Cancer Inst Monograph. 1966;19:1-27.
- Kannel WB, Schatzkin A. Sudden death: lessons from subsets in population studies. JAmCollCardiol. 1985;5:141B-149B.
- Cryer PE, Haymond MW, Santiago JV, Shah SD. Norepinephrine and epinephrine release and adrenergic mediation of smokingassociated hemodynamic and metabolic events. N Engl J Med.1976;295:573-577.
- WHO global report 2005 – Preventing chronic diseases: a vital investment. Geneva: World Health Organization; 2005
- World Health Organization. Report on the Global Tobacco Epidemic. Geneva, 2008; available at: http://www.who.int/tobacco/mpower/mpowerreport_full_2008.pdf (last day accessed 3 October 2013).
- S. Department of Health and Human Services. National Center for Chronic Disease Prevention and Health Promotion. Office on Smoking and Health. The Health Consequences of Smoking: A Report of the Surgeon General. Atlanta, 2004; available at: http://www.cdc.gov/tobacco/data_statistics/sgr/2004/complete_report/index.htm (last day accessed 22 September 2013).
- USA Institute of Medicine of the National Academies. Secondhand Smoke Exposure and Cardiovascular Effects: Making Sense of the Evidence. Washington DC: The National Academies Press, National Academy of Sciences, 2009; available at: http://www.nap.edu/catalog/12649.html (last day accessed 24 July 2013).
- Cellina GU, Honour AJ, Littler WA. Direct arterial pressure, heart rate, and electrocardiogram during cigarette smoking in unrestricted patients. Am Heart J. 1975;89:18-25.
- Dietz R, Schomig A, Kusterer K, Dart AM, Kubler W. Vasopressor systems during smoking in humans. Klin Wochenschr. 1984; 62(suppl II):11-17.
- Grassi G, Seravalle G, Calhoun DA, Bolla GB, Zanchetti A, Mancia G. Alterations in sympathetic nerve traffic during cigarettesmoking in man: a preliminary report. J Hypertens. 1991;9(suppl 6):S52-S53.
- Delius W, Hagbarth K-E, Hongell A, Wallin BG. Manoeuvres affecting sympathetic outflow in human muscle nerves.Acta Physiol Scand. 1972; 84:82-94.
- Mosqueda-Garcia R, Biaggioni I, Haile V, Robertson R, Robertson D. Effects of nicotine infusion on sympathetic nerve traffic and baroreflex responses in man. Clin Res. 1990;38:259.
- Mark, A. L., Victor, R. G., Nerhed, C. &Wallin, B. G. (1985). Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circulation Research.57: 461–469.
- Wallin, B. G., Victor, R. G. &Mark, A. L. Sympathetic outflow to resting muscles during static handgrip and post contraction muscle ischemia. American Journal of Physiology.1989;256: H105–110.
- Seals, D. R. & Victor, R. G.Regulation of muscle sympathetic nerve activity during exercise in humans. Exercise,Sports and Science Review.1991;19: 313–349.
- Victor, R. G., Bertocci, L. A., PRYOR, S. L. & NUNNALLY, R. L. Sympathetic nerve discharge is coupled to muscle cell pH during exercise in humans. Journal of Clinical Investigation. 1988; 82: 1301–1305.
- Sinoway, L., Prophet, S., Gorman, I., Mosher, T, Shenberger, J., Dolecki, M., Briggs, R. &Zelis, R. Muscle acidosis during static exercise is associated with calf vasoconstriction. Journal ofApplied Physiology.1989; 66: 429–436.
- Rowell, L. B. &O’leary, D. S. Reflex control of circulation during exercise: chemoreflexes and mechanoreflexes. Journal ofApplied Physiology1990;69: 407–418.
- Coote, J. H., Hilton, S. M. &Perez-Gonzalez, J. F). The reflex nature of the pressor response to muscular exercise. Journal of Physiology.1971; 215: 789–804.
- Mccloskey, D. I. &Mitchell, J. H. Reflex cardiovascular and respiratory responses originating in exercising muscle. Journal of Physiology. 1972;224: 173–186.
- Michael, E. L., Rummel, J. A., Sawin, C. F., Buderer, M. C. &Lem, J. D. Results of Skylab Medical Experiment M171 – metabolic activity. In Biomedical Results from Skylab, ed. Johnston, R. S. & Dietlein, L. F.National Aeronautics and Space Administration, Washington, DC, USA.1977; 372–387.
- Tibes, U. Reflex inputs to the cardiovascular and respiratory centers from dynamically working canine muscles: Some evidence for involvement of group III or IV nerve fibers. CirculationResearch. 1977; 41: 332–341.
- Singh N, Aggarwal AN, Gupta D, et al. Prevalence of low body mass index among newly diagnosed lung cancer patients in North India and its association with smoking status. Thoracic Cance .2011; 2:27-31.
- Benowitz NL, Gourlay SG. Cardiovascular Toxicity of Nicotine: Implications for Nicotine Replacement Therapy. J Am Coll Cardiol. 1997; 29:1422–1431.
- Seals DR. Influence of force on muscle and skin sympathetic nerve activity during sustained isometric contractions in humans. JPhysiol. 1993; 462: 147-59.
- MacDougall JD, Tuxen D, Dale D, Moroz J. Sutton J. Arterial blood pressure response to heavy resistance exercise. J Appl Physiol. 1985; 58: 785-90.
- Holly R. Middlekauff, MD,Jeanie Park, MD,Roya S. Moheimani, BS.. Adverse Effects of Cigarette and Noncigarette Smoke Exposure on the Autonomic Nervous System Mechanisms and Implications for Cardiovascular Risk.journal of the American college of cardiology. 2014 ; 64: 16,
- Rowell, L. B. & O’leary, D. S. Reflex control of circulation during exercise: chemoreflexes and mechanoreflexes. Journal ofApplied Physiology.1990;69: 407–418.
- Guyton A C, Hall J E. Textbook of Medical physiology.11th edition Newdelhi: Elsevier; 2007; pp 748-760, 872, 1022.
- BhatAN,Sadhoo AK,Yograj S,KaurG.Autonomic functions in postmenopausal women,JK SCIENCE.2005:17(3):135-139
- Mervi Kotamaki. Smoking induced differences in autonomic responses in military pilot candidates .Clinical Autonomic Research. 5(1):31-36.
- Alyan O, Kacmaz F, Maden O et al. Effects of smoking on heart rate variability and plasma n-terminal pro-b type natriuretic peptide in healthy subjects: is there the relationship between both the markers? Ann Noninvasive Electrocardiol.2008; 13(2):137-144.
- Sagawa K, Maughan L, Suga H, Sunagawa K. Cardiac Contraction and the Pressure Volume Relationship. New York: Oxford University Press, 1988; 171-232.







