Mahdis Motaghi1 and Parisa Ziarati*2
1Pharmaceutical Sciences Research Center, Pharmaceutical Sciences Branch, Islamic Azad University, Tehran-Iran (IAUPS).
2Department of Medicinal Chemistry, Pharmacy Faculty, Pharmaceutical Sciences Branch, Islamic Azad University (IAUPS), Tehran –Iran.
*Corresponding Author E-mail: ziarati.p@iaups.ac.ir
DOI : https://dx.doi.org/10.13005/bpj/998
Abstract
Removal of heavy metal ions from contaminated systems by agricultural and fruit and vegetable processing waste materials is an innovative and auspicious technology. The potential of the waste material on adsorbing heavy metals depends on the affinity, capacity and specificity including physico-chemical nature of it. In the current study the utilization of banana peel as food waste materials due to prospective metal bio-sorption capacity to toxic heavy metal ions: Cadmium and Lead from Oryza Sativa rice from Astaneh Ashrafieh, Gilan province in the north of Iran was investigated. The effect of soaking rinsed rice samples by NaCl and modified banana peel adsorbent by different concentration, pH, contact time and percentage of adsorbent and association of cooking methods on Cadmium and Lead contents were studied. Heavy metal contents in raw, rinsed, soaked by adsorbent and cooked and drained rice samples were determined by Atomic Absorption spectrophotometer. The minimum and maximum Cd and Pb contents in rinsing rice and cooked polished Oryza Sativa rice were 0.002 and 0.041 (mg/kg DW) in Iranian rice variety respectively . It was found that cooking rice by soaking rinsed rice samples by NaCl 2% and Banana peel modified by Citric Acid 0.5% at least for 1 hours had the greatest effect (significantly affect p<0.001) with regards to lowering Pb and Cd levels in cooked rice. Specifically, it preferentially reduced the Cadmium content by 93.2 % and Lead content by 83.78% from the raw rice, when combined with rinse washing and being soaked by salt for one hour contact time. Our finding shows high potential metal biosorption capacity of dried banana peel. The results of the current study suggest that banana peel waste modified by citric acid 1% can be used beneficially in treating rice containing heavy metal ions.
Keywords
Removal Heavy Metal; Banana peel; Oryza Sativa rice; Adsorbent
Download this article as:| Copy the following to cite this article: Motaghi M, Ziarati P. Adsorptive Removal of Cadmium and Lead from Oryza Sativa Rice by Banana Peel as Bio-Sorbent. Biomed Pharmacol J 2016;9(2). |
| Copy the following to cite this URL: Motaghi M, Ziarati P. Adsorptive Removal of Cadmium and Lead from Oryza Sativa Rice by Banana Peel as Bio-Sorbent. Biomed Pharmacol J 2016;9(2). Available from: http://biomedpharmajournal.org/?p=7500 |
Introduction
Recently scientific approach has been diverted towards the biomaterials which are byproducts or the wastes from large scale industrial operations and agricultural waste materials or even vegetable and food processing wastes. The major advantages of bio-sorption over conventional treatment methods include: low cost, high efficiency, minimization of chemical or biological sludge, no additional nutrient requirement, and regeneration of bio-sorbents and possibility of metal recovery. Agricultural materials particularly those containing cellulose shows potential metal bio-sorption capacity. The basic components of the agricultural waste materials biomass include hemicellulose, lignin, extractives, lipids, proteins, simple sugars, water hydrocarbons, starch containing variety of functional groups that facilitates metal complexation which helps for the sequestering of heavy metals [1-3]. Agricultural waste materials being economic and eco-friendly due to their unique chemical composition, availability in abundance, renewable, low in cost and more efficient are seem to be viable option for heavy metal remediation. Studies reveal that various agricultural waste materials such as rice bran, rice husk, wheat bran, wheat husk, saw dust of various plants, bark of the trees, groundnut shells, coconut shells, black gram husk, hazelnut shells, walnut shells, cotton seed hulls, waste tea leaves, Cassia fistula leaves, maize corn cob, sugarcane bagasse, apple, banana, orange peels, soybean hulls, grapes stalks, water hyacinth, sugar beet pulp, sunflower stalks, coffee beans, nut’s shells, cotton stalks , etc has been tried [ 4-16]. These promising agricultural waste materials are used in the removal of metal ions either in their natural form or after some physical or chemical modification. Food processing wastes are those end products of various food processing industries that have not been recycled or used for other purposes. They are the non-product flows of raw materials whose economic values are less than the cost of collection and recovery for reuse; and therefore discarded as wastes. These wastes could be considered valuable by-products if there were appropriate technical means and if the value of the subsequent products were to exceed the cost of reprocessing. Residues in this case cannot be regarded as wastes but become an additional resource to augment existing natural materials. Recycling, reprocessing and eventual utilization of food processing residues offer potential of returning these by-products to beneficial uses rather than their discharge to the environment which cause detrimental environmental effects. Successful food waste reprocessing involves (a) rendering recovered by-products suitable for beneficial use, (b) promoting marketability to ensure profitable operating, (c) employing reprocessing technology, and (d) creating an overall enterprise that is acceptable and economically feasible. Due to effective utilization of food residues to occur, food manufacturers could be invested in specialized secondary industry to utilize the residues [18-22].
Large amounts of fruit and vegetable processing wastes are produced from packing plants, canneries, etc., which may be disposed in several ways including immediate use for landfill or drying to a stable condition (10 % moisture) in order to use an animal feed during out of season, or which, alternatively, may be processed biotechnologically in order to produce SCP. First choice is not economical, and the second one is expensive due to drying cost. Industry continues to make progress in solving waste problem through recovery of by products and waste materials such as peel, pulp, or molasses by the employment of fermentation process. The other innovative and new approach of utilization of fruit waste is being as adsorbent of heavy metals from aquatic or other contaminated systems. Potential use of rice bran and wheat bran was tried for sequestering cadmium and significant removal efficiency was reported [23-25]. Studies were also conducted on use of rice polish, rice husk and black gram husk in their natural as well as modified form for the removal of cadmium and their relative efficiency was reported [25-28]. Bark of the plants such as Pecia glehnii and Abies sachalinensis and dried plant biomass of parthenium was tried for the removal of cadmium [29, 30]. Use of other parts of the plants such as peels of peas, fig leaves, broad beans, orange peels, medlar peels and jack fruits as adsorbents have been reported to show high removal efficiency at acidic pH [31].
Banana represents one of the most important fruit crops, with a global annual production of more than 50 million tons. Worldwide production of cooking bananas amounts to nearly 30 million tons per year. Peels constitute up to 30% of the ripe fruit. About 1000 banana plants are estimated to yield 20–25 tons of pseudostems providing about 5% edible starch. Attempts at utilization of banana waste include the biotechnological production of protein, ethanol, α-amylase, hemicellulases and cellulases. Very recently, anthocyanin pigments in banana bracts were evaluated for their potential application as natural food colorants. It was concluded that the bracts proved to be a good and abundant source of anthocyanins of attractive appearance, as well as being a useful tool in anthocyanin identification since all six most common anthocyanidins (delphinidin, cyanidin, pelargonidin, peonidin, petunidin and malvidin) are present. Most of the carotenoids found in banana peels were demonstrated to be xanthophylls esterified with myristate, and to a lesser extent with laurate, palmitate or caprate [17]. Efforts are needed to develop new technologies and to institute suitable measures to promote waste reclamation; this can only be achieved if food residues are considered as complementary resources rather than as undesirable wastes. Food industry produces large volumes of wastes, both solids and liquid, resulting from the production, preparation and consumption of food. These wastes pose increasing disposal and potential severe pollution problems and represent a loss of valuable biomass and nutrients. Beside their pollution and hazard aspects, in many cases, food processing wastes might have a potential for conversion into useful products of higher value as by-product, or even as raw material for other industries, or for use as food or feed after biological treatment. Banana peels, representing 40% of the total weight of fresh bananas [32]. The Use of principal component and cluster analysis to differentiate banana peel flours based on their starch and dietary fiber components have been underutilized. One way to utilize this waste is by converting the peel into banana peel flour (BPF) [33]. This product can be exploited further into new products that have a standardized composition and functional properties for various industrial and domestic uses [ 34-37],of particular interest, is the finding that banana peel extract contains higher levels of antioxidant compounds than the pulp [38] , promising a wider range of application of the peels in food and nutraceuticals. Rice, especially white rice, Oryza sativa L. is the staple food in the diet of various people including Asian countries [39-41]. Rice is the second food in high consumption among Iranian people. Half of the world populations consume rice as their main food [42]. It is the commonest crop grown in agricultural lands in the north of Iran [43]. Rice is the seed of the monocot plants (Oryza sativa), for example Asian rice or (Oryza glaberrima), for example African rice of the family, Graminaeae (grass family) [44]. Environmental contaminants are chemicals that are present in the environment in which the food is grown, harvested, transported, stored, packaged, processed, and consumed. .Although Iran is eleventh producer of rice at the world with an annual production 2600000 tons in 2010, during the last years the demand for rice has considerably been increased in comparison with its production; as a result, currently Iran is known as one of the large-scale importer of rice countries [39, 40, 45].
In this study rice was observed for its special individual consumption as a staple food in Iran [46] and probable hazards of its heavy metal contents on population health. Rice variety, treatment of rice and diversity of cooking may affect elemental content and intake of heavy metals [41, 47 -48]. The current study deals with the utilization of banana peel as agricultural and food waste materials as bio-sorbents for removal of toxic heavy metal ions: Cadmium and Lead from contaminated rice.
Material and Method
Bio-Sorbent
Two common banana varieties, Cavendish (M. acuminata L. cv. cavendishii) and Dream (M. acuminata collaAAA cv. Berangan) imported mostly from Ecuador, India and Philippine, bananas were purchased from 20 markets in Tehran –Iran. Yellow banana peels were chosen as adsorbent material. The fruits were washed with tap water and separated into pulp and peel. To reduce enzymatic browning, the peels were then dipped in a 1% (w/v) citric acid solution for 10 min, drained and dried in an oven at 100°C for 24 hours and homogenized in a blender to utilize in adsorption experiments.
Various banana peel (BP) forms were used, the first was as powder of dried pieces and the second was as carbon active formed from heated BP in 450 0c and finally powdered peels that treated by citric acid: 1 % w/v which sieved through 4mm stainless steel sieve. All peel forms were examined for bio-removal of both cadmium and lead from soaking rice samples under various factors such as pH, concentration of BP and contacting time.
Rice Sampling Method
40 samples of Iranian Oryza sativa rice were purchased randomly from 5 popular brands of recognized rice market in 2016 from Astaneh Ashrafieh, in Gilan province near to Caspian Sea, north of Iran (Figure 1).
5 portions of Oryza sativa packed in 10 kg portions were mixed before use. All experiments were conducted with 5 replications.
 |
Figure 1: Region of agriculture Astaneh Ashrafiyeh city
|
Experiment
Lead and Cadmium concentrations of Raw, Rinsed , soaked by water / NaCl 2% , soaked by BP( Banana peel powder) / NaCl 2% in different contact time, cooked in both states of treated and untreated by BP association by two different of cooking method ( drained and traditional method ) were determined by wet digestion method and using 10 g of each rice sample and 25 ml concentrated nitric acid ( 65% Merck) and 8 ml of Hydrochloric Acid ( 36.5%, Merck) was added and placed on a hot plate with gradual heat increase to insure full digestion and the disappearance of any residual and both lead and cadmium contents were determined by using flame atomic absorption spectrophotometer (FAAS).
Standardized international protocols were followed for the preparation of material and analysis of heavy metals contents by wet digestion method and atomic absorption spectrophotometer analysis based on annual book of ASTM standards and AOAC [48-51]. All digested sample flasks were firstly heated slowly and then vigorously till a white residue is obtained. The residue was dissolved and made up to 10 ml with 0.1 N HNO3 in a volumetric flask.
Blanks and samples were also processed and analyzed simultaneously. Blanks (10% v/v of nitric acid) and samples were also processed and analyzed simultaneously. All the chemicals used were of analytical grade (AR). This method has been followed in 4 stages for raw rice (untreated samples, rinsing rice, draining and cooked rice. The rinsing samples prepared by washing 5 times and in each step proportion of water and rice was 4:1 and for preparing of cooking rice bring the water to boil and then add 10 gram of oven-dried samples when the water has come to boil( rice is cooked in lots of water just like pasta) for 10 minutes then drain it in a colander and wash it by cold water just one time ( traditional method for preparing rice in Iran) [45] and for preparing draining rice samples put the oven-dried samples into boiling water and then heating the plate for 10 minutes till the steam escape . All draining and cooking rice samples have rinsed 5 times then followed by the procedure.
Bio-Removal Cadmium and Lead from Rice Samples
According to the results of all experiments applied above, current investigation was designed to examine the capacity of banana peels for the bio-removal of both cadmium and lead ions from contaminated rice samples after the determination of these metals in such rice samples in different states. In this experiment, 0.5 g powder of dried lemon peels into 3 forms of : as powder of dried pieces and the second was as carbon active formed from heated BP in 450 0c and finally powdered peels that treated by citric acid: 1 % w/v , were placed into plastic tank containing 50 g of rinsing rice samples ( 5 times by distilled water ) and 250 ml of deionized water left under laboratory conditions at pH = 6.5 and 250 C for almost 1 hour. Half of samples were not treated by Bp in order to find out the potential of bio-absorb.
Bio-sorbed metal concentration (mg/l) and bio-sorption capacity (%) were calculated by using the following equations [52-54]:
Bio-sorbed metal conc. (mg/l) = Ci – Cf
Bio-sorption capacity % = Ci – Cf / Ci X 100
Where Ci =initial metal concentration and Cf = final metal concentration.
Risk Assessment
To evaluate the potential risk of rice consumption containing the heavy metals, Provisional Tolerable Daily Intake (PTDI) for a 60kg adult person was calculated by the following equation in which C is the Arsenic concentration in rice, Cons is the average consumption of rice in country (110g per capita per day) and BW is body weight of an Iranian adult person (60kg). The output was compared with the WHO/FAO and Iranian standard level.
PTDI = C × Cons / Bw
The Iran standard PTDI limits have been recommended for, Cd, Pb and As 0.001, 0.0036 and 0.0021mg/day/kg Bw, respectively [39,41].
Statistical analysis
The values reported here are means of five values. Data were tested at different significant levels using student t-test to measure the variations between the concentration of banana peel and contact time parameters before and after treated by banana peel adsorbent . One way analysis of variance (One-ANOVA) was used for data analysis to measure the variations of heavy metal concentrations using SPSS 22.0 software (SPSS Inc, IBM, Chicago, IL).
Results and Discussion
Iran Standard (No. 12968) has established the maximum limit of Cd in rice about 0.06mg/kg and on the whole Institute of Standard and Industrial Re-search of Iran set limit of 0.15 mg/ kg as the maximum level for lead and arsenic and 0.06 mg/kg for cadmium in rice (Organization INS. Food & feed-maximum limit of heavy metals, in 2013. [55]. Although the concentration of Pb and Cd varied among the samples, 59.8% of the rice samples contained lower limit than the upper level of 0.15 and 0.06 mg/kg recommended by Iran Standard.
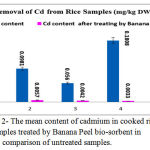 |
Figure 2: The mean content of cadmium in cooked rice samples treated by Banana Peel bio-sorbent in comparison of untreated samples.
|
The results of Cadmium and Lead contents in 880 samples of raw, rinsing, soaking by NaCl 2% , boiling – drained and cooking rice samples are shown in figures 2 and 3. All concentrations are expressed as mg /kg DW. Results show that the mean content of Cadmium and Lead in the most samples from samples is higher than maximum levels set by national standard and a few of them higher than maximum set by FAO/WHO. ANOVA analysis showed that there was a significant difference in Pb content in rinsing and raw and drained rice samples (p < 0.01 and p < 0.001 respectively). The maximum Pb content in draining- cooked rice and traditional cooked polished rice belong to brand 5 by 2.0141 in untreated rice by banana peel while this sample after soaking by BP powder and NaCL 2& after 1 hour remarkably being detoxified and Pb content decrease significantly to 0.5333 (mg/kg DW) which shows p < 0.05 .
The results in figure 3 revealed that all studied boiling – drained and cooked rice samples after treating by bio-absorbent BP in companion of salt (100%) had lead content less than maximum permissible level 0.15 mg/kg and in all untreated samples except brand 5samples the concentrations of cadmium were over than maximum level which is recommended by FAO/WHO Expert Committee on Food Additives and national standard [36,37,38], while treating by BP resulted in being in safe level.
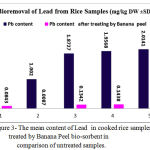 |
Figure 3: The mean content of Lead in cooked rice samples treated by Banana Peel bio-sorbent in comparison of untreated samples.
|
There was variation in the effectiveness of rinse and washing in removing heavy metals from raw rice (Table 1). The minimum and maximum Cd and Pb contents in rinsing rice and cooked polished Oryza Sativa rice were 0.002 and 0.041 (mg/kg DW) in Iranian rice variety respectively . It was found that cooking rice by soaking rinsed rice samples by NaCl 2% and Banana peel modified by Citric Acid 0.5% at least for 1 hours had the greatest effect (significantly affect p<0.001) with regards to lowering Pb and Cd levels in cooked rice. Specifically, it preferentially reduced the Cadmium content by 93.2 % and Lead content by 83.78% from the raw rice, when combined with rinse washing and being soaked by salt for one hour contact time.
Table 1: Lead and Cadmium concentrations, in studied Oryza sativa rice samples in various ways. Data are the averages of 3 replicates and 10 subsamples of 5 samples.
| State | Pb Content ± SE
( mg/kg DW ) |
Cd Content ± SE (mg/kg DW) |
| Raw Rice | 1.9065 ± 0.023 | 0.2016± 0.011 |
| Rinsed Rice ( 5 times) | 1.2380 ± 0.0141 | 0.1768 ±0.043 |
| Rinsed rice
4:1 Water: Rice and Soak with NaCl 2% |
1.1546 ± 0.011 | 0.1520 ±0.004 |
| Rinsed rice and Soak with Banana peel treated by citric acid 1% in companion of NaCl 2% ( 1 h) | 0.0682 ±0.0008 | 0.0021 ±0.0001 |
| Boiled Rice
after rinsing 4 times and soaking in salt solution for 1 hours |
1.0982 ±0.017 | 0.1428 ±0.0026 |
| Boiled Rice
after rinsing 4 times and soaking in presence of BP and salt solution for 1 hours |
0.0785 ± 0.0052 | 0.0042 ± 0.0002 |
| Cooked Rice
(Separating Water from untreated by BP Rice samples During Cooking Method) |
1.9891± 0.043 | 0.0834 ± 0.0012 |
| Cooked Rice
(Separating Water from Rice During Cooking Method) after soaking in presence of BP and salt solution for 1 hours |
0.0784 ± 0.0015 | 0.0050 ±0.0001 |
Rice absorbs heavy metals from the ground and water and this absorption is dependent on the rice type itself and content in the soil rather than on whether the rice is produced organically or not. Agricultural waste materials are usually composed of lignin and cellulose as the main constituents. Other components are hemicellulose, extractives, lipids, proteins, simple sugars, starches, water, hydrocarbons, ash and many more compounds that contain a variety of functional groups present in the binding process. Lignin is three dimensional polymer of aromatic compounds covalently linked with xylans in hardwoods and galactoglucomannans in softwoods [56]. The functional groups present in biomass molecules acetamido groups, carbonyl, phenolic, structural polysaccharides, amido, amino, sulphydryl carboxyl groups alcohols and esters [57]. These groups have the affinity for metal complexation. Some bio-sorbents are non-selective and bind to a wide range of heavy metals with no specific priority, whereas others are specific for certain types of metals depending upon their chemical composition. The presence of various functional groups and their com plexation with heavy metals during biosorption process has been reported by different research workers using spectroscopic techniques [56, 58 -59]. The presence of various functional groups and their complexation with heavy metals during biosorption process has been reported by different research workers using spectroscopic techniques [58-59].
Cadmium and Cadmium compounds as compared to other heavy metals are relatively water soluble therefore mobile in soil and tends to bio-accumulate. The long life time PVC-window frames, plastics and plating on steel are the basic sources of cadmium in the environment. Cadmium accumulates in the human body especially in kidneys, thus leading to dysfunction of the kidney [60]. Studies were also conducted on use of rice polish, rice husk and black gram husk in their natural as well as modified form for the removal of cadmium and their relative efficiency was reported [58]. Bark of the plants such as Pecia glehnii and Abies sachalinensis and dried plant biomass of parthenium was tried for the removal of cadmium [61]. Use of other parts of the plants such as peels of peas, fig leaves, broad beans, orange peels, medlar peels and jack fruits as adsorbents have been reported to show high removal efficiency at acidic pH [62]. Most of the studies showed that agricultural waste either in natural form or modified form is highly efficient for the removal of cadmium metal ions.
The major source of lead in the environment is from plastics, finishing tools, cathode ray tubes, ceramics, solders, pieces of lead flashing and other minor product, steel and cable reclamation. Lead can result in the wide range of biological effects depending upon the level and duration of exposure [12]. In the environment lead binds strongly to particles such as oil, sediments and sewage sludge so its removal is of great concern. Different agricultural wastes viz. rice straw, soybean hulls, sugarcane bagasse, peanut shells and walnut shells in their natural form have been used for removal of lead has been reported 98% [12]. Bankar and Dara in 1985 conducted studies on Febrifuga tree bark in its natural form. Petioler felt sheath palm (PFP), agro waste of black gram husk, flowers of Humulus lupulus, waste tea leaves and water hyacinth were studied for removal of lead and efficiency of these materials varies from 70 to 98% [12,57,58]. Lee et al. in 1999 investigated removal of lead and other metal ions by apple residues modified with phosphorous (V) oxychloride in both batch and column studies and compared the results. Rose petals pretreated with NaOH, calcium treated sargassum and sugarcane modified with succinic anhydride has also been utilized for significant removal of lead. Activated carbon prepared from agricultural waste was also explored by different workers and high effi- ciency for removal of lead has been reported [12,63]. Gupta et al. (1999) used bagasse fly ash for removal of lead with 65% removal efficiency. Saw dust of maple, Pinus sylvestries [12] and rubber wood saw dust has shown 85–90% removal efficiency but results show that modification did not enhance the removal efficiency for lead. Current study revealed that banana peel by citric acid 1% lead to reducing the Cadmium content by 93.2 % and Lead content by 83.78% from the raw rice, when combined with rinse washing and being soaked by salt for one hour contact time.
Bio-sorption is a relatively new process that has shown significant contribution for the removal of contaminants from aqueous effluents. In current study the toxic metal ion bio-sorption on inexpensive and efficient bio-sorbent- from agricultural and food & vegetable processing waste materials have been investigated as replacement strategy for existing conventional systems. The use of this low cost bio-sorbent is recommended since they are relatively cheap or of no cost, easily available, renewable and show highly affinity for heavy metals. As Literature also reveals that in some cases the modification of the adsorbent increased the removal efficiency, our finding proved that treating banana peel as a waste material by citric acid 1% could have high potential in removing lead and cadmium from rice as a stable food for most people in Iran and also others around the world. Other waste material from crop and vegetable processing and also agricultural wastes are recommended for future studies as very less work has been carried out in this direction.
Conflict of Interest
The authors have no affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this publication.
Acknowledgment
Financial Supports from Pharmaceutical Sciences Branch, Islamic Azad University (IAUPS) is gratefully acknowledged.
References
- Bailey, S.E., Olin, T.J., Bricka, R.M., Adrian, D.D. A review of potentially low-cost sorbents for heavy metals. Water Res., 1999; 33: 2469–79.
- Hashem, A., Abdel-Halim, E.S., El-Tahlawy, K.F., Hebeish, A.,. Enhancement of adsorption of Co (II) and Ni (II) ions onto peanut hulls though esterification using citric acid. Sci. Technol. ,2005; 23: 367–80.
- Hashem, A., Akasha, R.A., Ghith, A., Hussein, D.A.,. Adsorbent based on agricultural wastes for heavy metal and dye removal: A review. Energy Edu. Sci. Technol. ,2005; 19: 69–86.
- Annadurai, G., Juang, R.S., Lee, D.L. Adsorption of heavy metals from water using banana and orange peels. Water Sci. Technol., 2002; 47: 185–90.
- Cimino, G., Passerini, A., Toscano, G., Removal of toxic cations and Cr (VI) from aqueous solution by hazelnut shell. Water Res., 2000; 34: 2955–62.
- Hashem, A., Abou-Okeil, A., El-Shafie, A., El-Sakhawy, M. Grafting of high-cellulose pulp extracted from sunflower stalks for removal of Hg (II) from aqueous solution. Polym.-Plast. Eng., 2006; 45: 135–41.
- Hashem, A., Aly, A.A., Aly, A.S., Hebeish, A. Quaternization of cotton stalks and palm tree particles for removal of acid dye from aqueous solutions. Polym.-Plast. Eng., 2006; 45: 389–94.
- Macchi, G., Marani, D., Tirivanti, G. Uptake of mercury by exhausted coffee grounds. Environ. Lett., 1986; 7: 431–44.
- Mohanty, K., Jha, M., Biswas, M.N., Meikap, B.C. Removal of chromium (VI) from dilute aqueous solutions by activated carbon developed from Terminalia arjuna nuts activated with zinc chloride. Eng. Sci., 2005; 60: 3049–59.
- Orhan, Y., Bujukgungor, H. The removal of heavy metals by using agricultural wastes. Water Sci. Technol. ,1993; 28: 247–55.
- Reddad, Z., Gerente, C., Andres, Y., Ralet, M.-C., Thibault, J.-F., Cloirec, P.L. Ni (II) and Cu (II) binding properties of native and modified sugar beet pulp. Polym., 2002; 49: 23–31.
- Dhiraj, S., Garima, M., Kaur, M.P. Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions – A review. Bioresource Technology., 2008; 99 : 6017–27. Available online at sciencedirect.com .
- Alimardan, M., Ziarati, P., Jafari Moghadam, R. Adsorption of Heavy Metal Ions from Contaminated Soil by B. integerrima Barberry. Biomedical & Pharmacology Journal., 2016; 9(1): 169-75 .
- Ziarati, P., Alimardan, M. Study on Increasing Efficiency of Phytoremediation in Cadmium and Nickel Contaminated Soil. Chemistry Journal. 2015; 5(6), 86-92 .
- Ziarati, P., MirMohammad-Makki,F., Moslehishad, M. Novel Adsorption Method for Contaminated Water by Wild Endemic Almond: Amygdalus scoparia. BBRA., 2016;13(1): 147-53.
- Ziarati, P., Kermanshah,A., Moslehishad, M. Adsorption Heavy Metal from Contaminated Water by Modified Shell of Wild Endemic Almonds: Amygdalus lycioides and Amygdalus wendelboi . BBRA., 2015; 12(3): 2451-7.
- http://www.unido.org/fileadmin/import/32068_35FoodWastes.
- Anaç, D., H. Hakarer , İrget, M. Ege Üniv. Ziraat Fak. Dergisi., 1993;30: 3.
- Cooney, C, Rha, C., Tannenbaum, R. Food Res., 1980, 26: 1.
- Mahlia, T.M.I., Abdulmuin, M.Z. , Alamsyah, T.M.I. , Mukhlishien, D. An alternative energy source from palm oil wastes industry for Malaysia and Indonesia. Energy Conversation and Management., 2001; 42: 2109-18.
- Risse, M. Food waste composting, Public Service Associate, Biological and agricultural Engineering, The University of Georgia College of agricultural and Environmental Sciences. 2003.
- Schieber, A., Stintzing, F.C. , Carla, R., By –products of plant food processing as a source of functional compounds-Recent developments, Trends in Food Science and Technology.,, 2001;12: 401-13.
- Montanher, S.F., Oliveira, E.A., Rollemberg, M.C. Removal of metal ions from aqueous solutions by sorption onto rice bran. Hazard. Mater. B. , 2005;117: 207–11.
- Farajzadeh, M.A., Monji, A.B. Adsorption characteristics of wheat bran towards heavy metal cations. Purif. Technol. , 2004; 38: 197–207.
- Singh, K.K., Rastogi, R., Hasan, S.H. Removal of cadmium from waste water using agricultural waste using rice polish. Hazard. Mater. A. ,2005;121: 51–8.
- Iqbal, M., Saeed, A., Akhtar, N. Removal and recovery of lead II from single and multiple (Cd, Cu, Ni, Zn) solutions by crop milling waste (black gram husk). Hazard. Mater. , 2005;117: 65–73.
- Kumar, U., Bandyopadhyay, M. Sorption of Cd from aqueous solution using pretreated rice husk. Technol. 2006; 97: 104–9.
- Tarley, C.R.T., Arruda, M.A.Z. Biosorption of heavy metals using rice milling byproducts. Characterization and application for removal of metals from aqueous effl Chemosphere., 2004;54: 987–95.
- Ajmal, M., Rao, R.A.K., Ahmad, R., Khan, M.A. Adsorption studies on parthenium hysterophrous weed: Removal and recovery of Cd (II) from wastewater. Hazard. Mater. B. , 2006; 135: 242–8.
- Seki, K., Saito, N., Aoyama, M. Removal if heavy metal ions from solutions by coniferous barks. Wood Sci. Technol. , 1997;31: 441–7.
- Benaissa, H. Screening of new sorbent materials for cadmium removal from aqueous solutions. Hazard. Mater., 2006;132: 189–95.
- Tchobanoglous G., Theisen, , Vigil, S.A. Integrated solid waste management: Engineering principals and management issues. New York: McGraw-Hill; 1993;pp3–22.
- Ranzani, R., Sturion, G. L. , Bicudo, M. H. Chemical and bioloripe banana peel. Archivos Latinoamericanos de Nutricion ., 1996; 46(4): 320 -4.
- Bardiay, N., Somayaji, K., Khanna, S. Biomethanation of banana peel and pineapple waste . Bioresource Technology. , 1996; 58 (1): 73-6.
- Tewari, H. K., Marwaha, S. S., Rupal, K. Ethanol from banana peels. Agricultural Wastes . ,1986; 16: 135–46.
- Annadurai, G., Juang, R. S., Lee, D. J. Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. Journal of Hazardous Materials.,2002; 92: 263-74.
- Essien, J.P., Akpan, E. J. , Essien, E. P. Studies on mould growth and biomass production using waste banana peel. Bioresource and Technology., 2005; 96:1451-6.
- Someya, S., Yoshiki, Y., Okubo, K. Antioxidant compounds from Banana Musa cavendish). Food Chemistry ., 2002; 79(3): 351–4.
- Jafari- Moghadam, R., Ziarati, P. Reduction of Arsenic Content in Imported Polished Rice: Association of Cooking Method. Journal of Chemical and Pharmaceutical Research., 2016; 8(4):622-7.
- Ziarati,P., Azizi, N. Chemical Characteristics and Mineral Contents in Whole rice grains, Hulls, Brown rice, Bran and Polished Ali Kazemi Rice in Gilan province – North of Iran Intl J Farm & Alli Sci ., 2014; 2 (24): 1203-9.
- Ziarati, P., Arbabi, S., Arbabi-Bidgoli, S., Qomi, M. Determination of Lead and Cadmium Contents in ( Oryza Sativa) rice samples of agricultural areas in Gilan-Iran. Intenational Journal of Farming & Allied Sciences.,2013; 2 (11): 268 -71.
- Huang, S.Q., Peng, J., Qiu, C.X., Yang, Z.M. Journal of Inorg. Biochem., 2009;103(2): 282-7.
- Rabbani, D., Mostafaii, Gh. R., Dehghani, R., Gilasi, H., Hosein Abadi, Z. International Achieves if Health Sciences., 2015; 2(1): 25-9.
- Hoseini, M., Mafton, M., Karimian, N.A., Ronaghi, A.M., Emam, Y., Iranian J. Agricul. Sci. 2005; 36:869.
- Jafari Moghadam, R., Ziarati, P., Salehi Sormaghi, M.H., Gomi, M. Comparative Perspective to the Chemical Composition of imported Rice: Association of Cooking Method. Biomedical & Pharmacology Journal., 2015; 8(1): 149-55.
- Tuzen, M., Soylak, M. Food Chemistry. ,2007; 102: 1089-95.
- Naseri, M., Rahmanikhah, Z., Beiygloo, V., Ranjbar, S. Journal of Chemical Health Risks .,2014;4: 65–72.
- A.O.A.C. 1998. The association of analytical communities focuses on method validation and laboratory quality assurance. Official methods of analysis 16th edition, 4th revision. vol.1, chapter 9.
- ASTM. 2000. Annual Book of ASTM standards, water and Environmental technology. Standard Guide for preparation of Biological samples for inorganic chemical Analysis, Vol. 11.01, D 4638-95a (Reapproved 1999).
- A.O.A.C. 2000. Official method of analysis 17th edition, Horowitz edition intern, Maryland, USA. Vol. 1, 452- 456.
- Ziarati, P., Ziarati, N.N., Nazif, M.H., Khandehrouy, M. Oriental Journal of Chemistry., 2015; 31 (Spl Edn): 113- 20.
- Ziarati ,P . Determination of Contaminants in Some Iranian Popular Herbal Medicines. J Environment Analytic Toxicol., 2012; 2: 120. doi:10.4172/2161-0525.1000120.
- Husoon, A., Al-Azzawi, M.N.A., Al-Hiyaly, S.A.K. Investigation Biosorption Potential of Copper and Lead from Industrial Waste Water Using Orange and Lemon Peels. Journal of Al-Nahrain University., 2013; 16 (2): 713-9.
- Fahad, H. G. A study of efficiency of different microorganisms in thorium sorption from aqueous solutions. M.Sc. thesis. College of Science, Baghdad University, Iraq. 1994.
- Iranian National Standardization Organization; 2013. Available from: http://isiri.org/portal/File/ShowFile.asp.
- Garg, U.K., Kaur, M.P., Garg, V.K., Sud, D. Removal of hexavalent Cr from aqueous solutions by agricultural waste biomass. Hazard. Mater. ,2007; 140: 60–8.
- Gupta, V.K., Ali, I. Utilization of bagasse fly ash (a sugar industry waste) for the removal of copper and zinc from wastewater. Separation and Purification Technol., 2000;18: 131–40.
- Ahluwalia, S.S., Goyal, D. Removal of heavy metals from waste tea leaves from aqueous solution. Life Sci., 2005;5: 158–62.
- Tarley, C.R.T., Arruda, M.A. Z. Biosorption of heavy metals using rice milling byproducts. Characterization and application for removal of metals from aqueous effluents. Chemosphere ., 2004;54: 987–95.
- Volesky, B., Holan, Z.R. Biosorption of heavy metals. Progr., 1995;11: 235–50.
- Ajmal, M., Rao, R.A.K., Ahmad, R., Khan, M.A. Adsorption studies on parthenium hysterophrous weed: Removal and recovery of Cd (II) from wastewater. Hazard. Mater. B., 2006; 135: 242–8.
- Benaissa, H. Screening of new sorbent materials for cadmium removal from aqueous solutions. Hazard. Mater. ,2006;132: 189–95.
- Wilson, W., Yang, H., Seo, C.W., Marshall, W.E. Select metal adsorption by activated carbon made from peanut shells. Technol., 2006; 97: 2266–70.







