Mohammad Golbashy1, Hossein Sabahi1*, Iraj Allahdadi2, Hossein Nazokdast3 and Morteza Hosseini1
1Department of Life Science Engineering, Faculty of New Sciences and Technologies, University of Tehran, Tehran, Iran. 2Department of Agronomy, College of Aburaihan, University of Tehran, Tehran, Iran. 3Department of Polymer Engineering and Color Technology, University of Technology, Tehran Polytechnic, Tehran, Iran. Correspomding Author Email: hsabahi@ut.ac.ir
DOI : https://dx.doi.org/10.13005/bpj/950
Abstract
Among constituents of plants, polyphenols have received a great deal of attention, in recent years, due to their diverse biological functions. The effects of bulk polyphenols are limited by their relative low bioavailability and short half-life due to their varying structures, moderate solubility, and fast oxidation under basic conditions. Therefore, use of a polyphenol-loaded nanoparticles system suggested controlling polyphenols properties. As a proof-of-concept study, the pomegranate peel polyphenol was loaded into montmorillonite (MMT) clay to evaluate its utility as a drug delivery vehicle. Loading is complete within one hour and the most amounts of polyphenol that can be loaded into the clay are based on the polyphenol solution concentration in which the MMT is suspended. MMT showed a basal distance (d001) of 12.3 Å, which in the presence of pomegranate peel polyphenols increased to 15.34 Å, indicating a high degree of interlayering of these materials. This expansion confirmed the almost 100% intercalation. Analyses performed by TG also confirmed the effectiveness of this simple process to intercalate the polyphenol into clay lamellae. The results also suggest that the montmorillonite species, used in the current study, in 1:4 MMT/PP ratio and 1 hr stirrer, have desirable ecological and economic efficiency in production of polyphenol clay composites. This experiment results showed that due to its strong natural hydrophilicity, the montmorillonite can be a more suitable reservator and carrier for applications such as cosmetic than drug delivery in systemic circulation.
Keywords
Pomegranate; Phenolic compounds; Drug delivery system; Intercalation
Download this article as:| Copy the following to cite this article: Golbashy M, Sabahi H, Allahdadi I, Nazokdast H, Hosseini M. Synthesis The Montmorillonite- Pomegranate (Punicagranatum L.) Peel Polyphenols Nanostructure As A Drug Delivery Vehicle. Biomed Pharmacol J 2016;9(1) |
| Copy the following to cite this URL: Golbashy M, Sabahi H, Allahdadi I, Nazokdast H, Hosseini M. Synthesis The Montmorillonite- Pomegranate (Punicagranatum L.) Peel Polyphenols Nanostructure As A Drug Delivery Vehicle. Biomed Pharmacol J 2016;9(1). Available from: http://biomedpharmajournal.org/?p=6812 |
Introduction
Pomegranate (Punicagranatum L.) belongs to the Punicacea family and its fruits have achieved great attention for its health benefits in the last years[1]. It is widely grown in warm climate areas of South East Asia, the Mediterranean, the Americas and other parts of the world. Fruits, peels and roots of pomegranate have been commonly used in herbal remedies by local healers in many countries[2]. They have been used traditionally for their medicinal properties as anticancer, anti-inflammatory, anthelminthic and for other purposes such as tanning, dyeing and heavy metal removal[3].
Plant based diets rich in phytochemicals have been associated with a reduced risk of diseases such as certain types of cancer, inflammation, cardiovascular and neurodegenerative diseases[4]. Many investigators have reported that pomegranate has a free radical scavenger and potent antioxidant capacity that are attributed to the antioxidative properties of pomegranate polyphenols and sugar containing polyphenolic tannins and anthocyanin[5].
Other studies had showed the inhibitory effects of polyphenols on bacteria growth that are depending on bacteria species. Under the name of Pomegranate polyphenols, numerous compounds of different chemical structures are existence. In total, 48 compounds were detected by Fischer et al. [4] among which 9 anthocyanins, 2 gallotannins, 22 ellagitannins, 2 gallagyl esters, 4 hydroxybenzoic acids, 7 hydroxycinnamic acids and 1 dihydroflavonol were identified based on their UV spectra and fragmentation patterns in collision-induced dissociation experiments. Anthocyanin and tannin along with ellagic acid are the major phenolic compounds of pomegranate[6]. Figure 1 summarizes some important constituents of the pomegranate and its extracts.
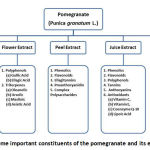 |
Figure 1: Some important constituents of the pomegranate and its extracts |
The effects of bulk polyphenols (e.g., anti-inflammatory, antitumoral, anti-aging) are limited by their relative low bioavailability and short half-life due to their varying structures, moderate solubility, and fast oxidation under basic conditions. Therefore, one option is to use polyphenol-loaded nanoparticles to better control polyphenols properties (bioavailability, pharmacokinetics, targeting, (photo-) stability, controlled release) and effects (e.g., “nano-chemoprevention,” antioxidation).
In recent years, attention has been focused on various Nano-adsorbent that have suitable adsorption capacities and are able to adsorb biologically polyphenol compound or other similar molecules at low-cost[7]. There has also been growing interest in the use of clays in drug delivery as slow release vehicles. Montmorillonite is one of the most interesting and widely investigated clays for molecules adsorption[8]. The structure of montmorillonite[9] consists of layers made up of one octahedral alumina sheet sandwiched between two tetrahedral silica sheets. Stacking of the silicate layers leads to a regular van der Waals gap between the layers. These clay platelets are over two orders of magnitude thinner, typically 1 nm in thickness[10]. Their surface properties and stability have been extensively studied. Within the inter-layer spaces, the charge compensating counter ions (K+, Na+, Ca2+ and etc.) are located that render hydrophilic clays. Depending on the compatibility level between clay and molecules, the systems exhibit intercalated or exfoliated structure. The intercalated structure corresponds to insertion of molecules between the layers of the clay. In the exfoliated (or delaminated) structure, the layers are separated independently[11]. Phenolic compounds can be stabilized by the intercalation into montmorillonite. Evidence has shown that some organic polyphenols and drugs are intercalated in interlayer spaces of clays and also adsorbed on the external planar surfaces [12-14]. However, studies on organic polyphenols extraction and adsorption onto montmorillonite structure are to date very limited. The objective of this study was to evaluate the raw montmorillonite interlayer capacity for pomegranate peel phenolic contents adsorption, which would be useful for drug delivery systems.
In this paper we report the first investigation of naturally occurring clay as a slow release delivery vehicle for Pomegranate (Punicagranatum L.) peel polyphenol (PP). Factors affecting the loading capacity of polyphenols into MMT including time, components ratio and polyphenol concentration were investigated using UV–Vis spectrophotometry. The slow release properties of the PP-MMT complex were examined using dispersion methods.
In the future, In-vitro studies would help in understanding the antimutagenicity and antimicrobial activity roles composite have on biologic organism.
Materials and Methods
Raw materials
Ripened pomegranates (P. granatum L.) were obtained from Ferdows of Iran. The peel and pulp were separated manually. The peels were washed with distilled water and air-dried till equilibrium humidity, then ground and transferred to darkness for further use. The raw montmorillonite clay (MMT) used as basis for the nanocomposite preparation was without purification (local sodium Bentonita, produced by Poodrsazan, Tehran, Iran). The chemical composition was SiO2: 61.03, Al2O3: 14.59, Fe2O3: 2.09, CaO: 0.77, MgO: 2.22, Na2O: 2.04, SO2: 0.37, Cl: 0.46, K2O: 0.76, TiO2: 0.22, BaO: 0.11 and Loss of ignition, 13.2.
Polyphenol Extraction
The ground peels were cut into pieces and extracted with water (DW). The solid/liquid ratio was 1/10 (w/v). The extracts were filtered through Whatman No. 41 filter paper. The resulting solvents were centrifuged to obtain supernatant and the residues were subject to extraction twice under the same condition[15]. All the supernatants collected were combined and then evaporated under vacuum at 50◦C., and the concentrates were powdered and stored in a desiccator[16].
Determination of total phenolic content
The total polyphenolic content of pomegranate extracts was determined UV-Vis spectrophotometrically using the Folin-Ciocalteu (FC) reagent according to the procedure explained by[17]. Briefly, 100 µL of phenol extract were mixed with 100 µL of FC reagent and allowed to stand at room temperature for 4 min; 800 µL of Na2CO3 5% were added to the mixture. After 20 min at 40◦C, the absorbance was measured at 750 nm. Results were expressed as gallic acid equivalents (mg of gallic acid per liter of solution).
Nanocomposite preparation
1 g of raw montmorillonite was dispersed in 150 mL of pomegranate peel extract solution at initial concentration of 10 and 40 g/L (with sealed cape). The temperature was kept at 25◦C. Then the suspensions were shaken with a speed of 200 rpm for 1 h. All experiments were run in triplicate to ensure reproducibility. All Samples were performed by centrifuging at 6000 rpm for 15 min, and then the sediments were dried in freeze dryer at -60 oC for 24 h.
Nanocomposite characterization
The intercalated composite was evaluated by two independent methods: the X-ray powder diffraction (XRD) and Thermo-gravimetric analysis (TGA).
– XRD analysis
The crystallization of materials was evaluated using a Philips Analytical XRD to probe the interlayer structure. The relative intensity was registered in a diffraction range (2θ) of 1−11° using a CO Kα incident beam.
– Thermal analyses
The thermal gravimetric analyses (TG) were carried out at a heating rate of 10°min-1 from roomtemperature to 800 °C using thermal gravimetric analyzer.
Desorption experiments
A test in aqueous medium was performed, adapted from[18]. In summary 2.5 g of modified montmorillonite were dispersed in 50 mL of deionized water and shaken at 500 rpm for 90 minutes at 37 °C. At specific time intervals, 1mL of the mixture was drowned by a syringe, filtered, and analyzed spectrophotometrically at 260 nm to determine the amount of compound released in solution. This procedure was repeated three times, and the amount of polyphenol released was determined by spectrophotometrically using the Folin-Ciocalteu (FC) reagent according to the procedure explained by[17].
Result and Discussion
Pomegranate peel polyphenols adsorption
The adsorbing effect of montmorillonite on different concentrations of pomegranate peels polyphenol showed that MMT could adsorb polyphenols in a concentration-dependent manner.
According to modified MMT weight differences, the adsorption percentages of MMT at 1 and 4 g polyphenol were 40 and 56 percentage respectively. There was a rapid saturation for both ratios; after 60 min, no further change could be observed in the adsorption capacity of MMT. For this reason, the adsorption time used for the composite synthesis was 1 h. The adsorption isotherm of the compound was also studied. Fig. 2 shows the adsorption isotherms of PP and Langmuir model linear transformation plot.
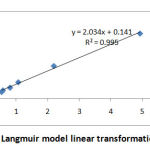 |
Figure 2: Langmuir model linear transformation plot. |
There was a steep increase in adsorption capacities with the initial concentration of adsorbate at low concentration. This was followed by the formation of a plateau at high concentrations, indicating the saturation of the adsorption sites of the materials. The Langmuir model was used to fit the experimental points and the constants related to this model are summarized in Table 1. There was an excellent correlation (R2 greater than 0.99), indicating the pertinence of this model for these systems. Consequently, it can be deduced that the adsorbed anions did not form a multilayer in the interlayer space and that all the adsorption sites were equivalent[19].
Table 1: Langmuir constant for the adsorption of pomegranate peel polyphenol
| Qm | KL | R2 |
| 7.07 | 0.069 | 0.99 |
XRD
To monitor the exfoliation and intercalation of polyphenol into clay after processing, the materials were analyzed by XRD (Fig. 3). As shown in Figure 3, the position of the d001 peak (2θ=8.33°) showed a substantial shift to low angle (2θ= 7.08° for 1MMT/1PP and 2θ= 6.68° for 1MMT/4PP) suggesting a change in the interlayer spacing of the clay. The interlayer expansion with d=12.3 A° for Mt and d=14.48 A° for 1gr PP+MMT, d=15.34 for 4gr PP+MMT, was observed to be about more than 2 and 3 A°. Increased surface loading of polyphenol, results in increasing of the interlayer space to 15.34 Å, indicating the almost 100% intercalation.
MMT showed a basal distance (d001) of 12.3 Å and according to the size of polyphenol (2.5 Å) [20] it can be concluded that pomegranate peel polyphenols increased basal distance to 15.34 Å, indicating a high degree of intercalation of these materials. The Height of XRD curves showed that the 1MMT/4PP composite peaks are much higher than 1MMT/1PP composite.
 |
Figure 3: X-ray diffraction patterns of Mt (a), 1Mt/1PP nanocomposite (b) 1Mt/4PP nanocomposite (c). |
These results are comparable to Dedzo and Detellier (2014) study[19]. These workers expanded the interplanar spacing of kaolinite by about 5.2 A° but by the very expensive mechanochemical technique. In our method the high polarity of polyphenols allows its insertion by weakening the hydrogen bonds between adjacent layers, while maintaining the cohesion and the stacking along the c-axis of layered clays[19]. Letaief and Detellier[21] in the same preparation technique reported an increase in interlayer spacing from 11.1 A° to 13.2 A° in response to modification. This higher increase in interplanar space of MMT in our study may be due to larger polyphenol molecules size or its more polarity molecules.
Amarasinghe et al. (2009) studied the mechanisms by which different fluids of varying polarities enter into the clay interlayer. Results showed a very good correlation between the polarity of the fluids and the d001 spacing of the MMT-solvent samples.
We can conclude that the species of MMT has caused to achieve high intercalation.
FTIR
The FTIR spectrum for montmorillonite shows absorption band at around 3627 cm-1 that attributed to O-H stretching Al-OH and Si-OH of octahedral and tetrahedral layers in Mt. The 3443 cm-1 and 1639 cm-1 band can be attributed to the H-O-H stretching and bending-in-plan vibration of interlayer water. A strong band at 1038 cm-1 is related to Si-O stretching that is highest in clay.
Comparing spectra between 1Mt/1PP and 1Mt/4PP composites shows more intercalation of polyphenols into interlayer of clay at 1Mt/4PP. The evidence for this claim is the lack of 3443 and 3627 cm-1 band of Mt at Mt/4PP nanocomposite, which are related to the H-O-H stretching and bending-in-plan vibration of interlayer water and phenolic O-H stretching of PP. This can indicates the formation of hydrogen bonds between OH moiety of PP with Al-O of octahedral sheet. Presence of more sharped bands at 1717 and 1616,1455,1354 and 624-1010 cm-1 in 1Mt/4PP nanocomposite which are related to carboxylic group C=O, C=C stretching vibration of aromatic ring, -CH- bending vibration of benzene ring and C-O stretching of carboxylic group, indicating higher surface banding between PP and surface of Mt.
 |
Figure 4: FTIR spectra of Mt (a) and 1Mt/4PP composite(b). |
TGA
Thermogravimetry was used to evaluate the thermal stability of MMT and PP intercalated/absorbed samples during thermal decomposition of solid materials. The thermogravimetric (TG) curves of MMT and MMT-PP are presented in Fig. 5(a) and (b) respectively. Between 30 °C and 100 °C, there was a significant weight loss (more than 3%) associated to water molecules trapped in the interlayer of MMT. The low temperature at which the thermal phenomenon arises suggests that the water molecules were retained by weak interactions (physisorption). The presence of the ionic liquid increases considerably the hydrophilicity of MMT. According to obtained results the adsorbed and intercalated PP is liberated in the second mass loss step (> 210 and < 630 °C). It is important to note that the final residue after 800 °C (~ 64%) corresponds to the initial Mt content expected. Several studies have reported the tendency of water molecules to establish hydrogen bonds (Wang et al., 2010). In this case, the highly polar environment of montmorillonite interlayer also provides more adsorption sites for polyphenols molecules. Mass losses occurring at higher temperatures accounted for the pyrolysis of organic matter remained trapped between the layers of the mineral after partial decomposition.
 |
Figure 5: TGA curves of MMT (a) and MMT/PP(b). |
Conclusion
The combination of the results of this study with the previous researches indicates that Montmorillonite could be effectively used for the intercalation of the Polyphenol derived from a simple one step extraction method of Pomegranate peel with water. Accordingly, we named the made nanocomposie eco-friendly which is comparable with several other high-cost, high energy and high reagent demand manners. XRD, FTIR and TGA analysis also confirm the higher intercalation of Pomegranate peel polyphenols. Moreover, the electrostatic forces, hydrogen bonding, ligand exchange and cation bridge may be dominative in the adsorption of polyphenols by clay minerals. This research showed that Montmorillonite can be a more suitable reservator and carrier for application such as cosmetic than drug delivery in systemic circulation.
Reference
- D. E. Ilham Hmid, Hafida Hanine, Ahmed Oukabli, Emira Mehinagic, “Comparative study of phenolic compounds and their antioxidant attributes of eighteen pomegranate (Punica granatum L.) cultivars grown in Morocco,” Arabian Journal of Chemistry, p. Article in Press, 2013.
- N. S. Al-Zoreky, “Antimicrobial activity of pomegranate (Punica granatum L.) fruit peels,” International Journal of Food Microbiology, vol. 134, pp. 244-248, 2009.
- H. Saad, F. C.-E. Bouhtoury, A. Pizzi, K. Roded, B. Charrier, and N. Ayeda, “Characterization of pomegranate peels tannin extractives,” Industrial Crops and Products, vol. 40, pp. 239-246, 2012.
- U. A. Fischer, R. Carle, and D. R. Kammerer, “Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD–ESI/MSn,” Food Chemistry, vol. 127, pp. 807-821, 2011.
- P. S. Negi, G. K. Jayaprakasha, and B. S. Jena, “Antioxidant and antimutagenic activities of pomegranate peel extracts,” Food Chemistry, vol. 80, pp. 393-397, 2003.
- G. Mousavinejad, Z. Emam-Djomeh, K. Rezaei, and M. H. H. Khodaparast, “Identification and quantification of phenolic compounds and their effects on antioxidant activity in pomegranate juices of eight Iranian cultivars,” Food Chemistry, vol. 115, pp. 1274–1278, 2009.
- A. D. Martino, M. Iorio, P. D. Prenzler, D. Ryan, H. K. Obied, and M. Arienzo, “Adsorption of phenols from olive oil waste waters on layered double hydroxide, hydroxyaluminium–iron-co-precipitate and hydroxyaluminium–iron–montmorillonite complex,” Applied Clay Science, vol. 80-81, pp. 154-161, 2013.
- E. I. Pereira, F. B. Minussi, C. C. T. da-Cruz, A. C. C. Bernardi, and C. Ribeiro, “Urea−Montmorillonite-Extruded Nanocomposites: A Novel Slow-Release Material,” Journal of Agricultural and Food Chemistry, vol. 60, pp. 5267-5272, 2012.
- K. Faghihi, F. Shabani, and M. Shabanian, “Synthesis of New Poly(ether-imide) Nanocomposite Containing Bicyclo Segments by Solution Intercalation,” Journal of Macromolecular Science, Part A: Pure and Applied Chemistry, vol. 48, pp. 381-386, 2011.
- Y. Zhang and J. R. G. Evans, “Approaches to the manufacture of layered nanocomposites,” Applied Surface Science, vol. 258, pp. 2098-2102, 2012.
- M. J. Sanchez-Martin, M. S. Rodriguez-Cruz, M. S. Andrades, and M. Sanchez-Camazano, “Efficiency of different clay minerals modified with a cationic surfactant in the adsorption of pesticides: Influence of clay type and pesticide hydrophobicity,” Applied Clay Science, vol. 31, pp. 216-228, 2006.
- G. V. Joshi, B. D. Kevadiya, H. A. Patel, H. C. Bajaj, and R. V. Jasra, “Montmorillonite as a drug delivery system: Intercalation and in vitro release of timolol maleate,” International Journal of Pharmaceutics, vol. 374, pp. 53-57, 2009.
- R. I. Iliescu, E. Andronescu, C. D. Ghitulica, G. Voicu, A. Ficai, and M. Hoteteu, “Montmorillonite–alginate nanocomposite as a drug delivery system–incorporation and in vitro release of irinotecan,” International Journal of Pharmaceutics, vol. 463, pp. 184– 192, 2014.
- M. Ghadiri, W. Chrzanowski, W. H. Lee, A. Fathi, F. Dehghani, and R. Rohanizadeh, “Physico-chemical, mechanical and cytotoxicity characterizations of Laponite®/alginate nanocomposite,” Applied Clay Science, vol. 85, pp. 64–73, 2013.
- C. Wang, L. Shi, L. Fan, Y. Ding, S. Zhao, Y. Liu, et al., “Optimization of extraction and enrichment of phenolics from pomegranate (Punica granatum L.) leaves,” Industrial Crops and Products, vol. 42, pp. 587– 594, 2013.
- Y. Li, C. Guo, J. Yang, J. Wei, J. Xu, and S. Cheng, “Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract,” Food Chemistry, vol. 96, pp. 254-260, 2006.
- G. Gambacorta, M. Faccia, A. Trani, C. Lamacchia, and T. Gomes, “Phenolic composition and antioxidant activity of Southern Italian monovarietal virgin olive oils,” European Journal of Lipid Science and Technology, vol. 114, pp. 958–967, 2012.
- L. Zampori, P. G. Stampino, C. Cristiani, P. Cazzola, and G. Dotelli, “Intercalation of poly(ethylene-oxides) in montmorillonite: Tailor-made nanocontainers for drug delivery systems,” Applied Clay Science, vol. 50, pp. 266-270, 2010.
- G. K. Dedzo and C. Detellier, “Intercalation of two phenolic acids in an ionic liquid–kaolinite nanohybrid material and desorption studies,” Applied Clay Science, vol. 97-98, pp. 153–159, 2014.
- D. Dorniani, A. Kura, S. H. Hussein-Al-Ali, M. Z. B. Hussein, S. Fakurazi, A. H. Shaari, et al., “In Vitro Sustained Release Study of Gallic Acid Coated with Magnetite-PEG and Magnetite-PVA for Drug Delivery System,” The Scientific World Journal, vol. 2014, pp. 1-11, 2014.
- S. Letaief and C. Detellier, “Reactivity of kaolinite in ionic liquids: preparation and characterization of a 1-ethyl pyridinium chloride–kaolinite intercalate,” Journal of Materials Chemistry, vol. 15, pp. 4734–4740, 2005.








