Fatemeh Mehrarad 1, Parisa Ziarati*2 and Zahra Mousavi3
1Pharmacy Faculty, Pharmaceutical Sciences Branch, Islamic Azad University (IAUPS), Tehran, Iran. 2Department of Medicinal Chemistry, Pharmaceutical Sciences Branch, Islamic Azad University (IAUPS), Tehran, Iran. 3Department of Pharmacological and Toxicology, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran-Iran
DOI : https://dx.doi.org/10.13005/bpj/922
Abstract
This investigation was carried out to determine the accumulation of heavy metals in plarganium grandiflorum grown in chemical and toxicology laboratories’ pharmaceutical effluent and wastewater irrigated soil in the vicinity of sewage treatment plant (STP), Pharmacy Faculty, Tehran. The results revealed that wastewater was highly rich in plant nutrients and heavy metals. The wastewater irrigation significantly (P<0.05/ P<0.01) increased the contents of heavy metals in the soil and plants grown in wastewater irrigated soil. The enrichment of various metals were recorded in the order of Fe >Zn > Pb >Cu > Cd > Cr > Mn in these plants. Moreover contents of different heavy metals in the different parts of plants such as root, young and old leaves and stems showed significant (P<0.05) and positive correlation with contents of Cd (r = +82 to r = +96), Cr (r = +74 to r = +94), Cu (r = +84 to r = +98), Fe (r = +88 to r = +98), Mn ( r = +80 to r = +96), Pb (r = +74 to r = +96) and Zn (r = +88 to r = +98) in the wastewater irrigated soil. Although, the contents of Cd, Cu, Fe, Mn, Pb and Zn in soil after growing of plarganium grandiflorum after 60 days were recorded within the prescribed limit of WHO/FAO standards. Pelargonium (Grandiflorum) has shown ability to extract lead, Cr (III) , Cr(VI) and Cd from contaminated soils; about 35.9 %, 41.9% , 41.7% and 29.8% respectively in the root and leaves zone. To quantify the occurrence and the distribution of heavy metals and to prevent them from passing through wastewater collection and treatment systems into soil and ground water bodies represents an urgent task for applied environmental sciences in the coming years. Public acceptance of green technologies is generally higher than that of industrial processes. The responsible organizations should stimulate research to upgrade existing waste water treatment by implementing phytoremediation modules and demonstrating their reliability to the public.
Keywords
Contamination; Heavy metals; plarganium grandiflorum ;Pharmaceutical effluent
Download this article as:| Copy the following to cite this article: Mehrarad F, Ziarati P, Mousavi Z. Removing Heavy Metals from Pharmaceutical Effluent by Plarganium Grandiflorum. Biomed Pharmacol J 2016;9(1) |
| Copy the following to cite this URL: Mehrarad F, Ziarati P, Mousavi Z. Removing Heavy Metals from Pharmaceutical Effluent by Plarganium Grandiflorum. Biomed Pharmacol J 2016;9(1). Available from: http://biomedpharmajournal.org/?p=6838 |
Introduction
There is a lack of toxicity studies for various groups of chemicals, for example: pharmaceuticals [1-3], nano-particles [4-5] and industrial chemicals [6-8]. This has resulted in difficulties when performing risk assessments since any risk assessment relies on the availability of reliable and relevant studies. Pharmaceuticals were first identified to pose environmental risks in the 1990s, and since then the number of available monitoring and effect studies has increased steadily. Today, several hundred active pharmaceutical ingredients (APIs) have been found in sewage water, surface water, groundwater, soil, air, or biota in concentrations from sub-ng/L to more than μg/ L. [9-11] Thus far, there are several examples of APIs convincingly shown to cause effects on organisms in the environment. Pharmaceuticals are synthetic or natural chemicals that can be found in prescription medicines, over-the-counter therapeutic drugs and veterinary drugs which contain active ingredients that have been designed to have pharmacological effects and confer significant benefits to society. They include painkillers, birth control pills, tranquilizers and anti-depressants [12] among many others. Waste from pharmaceuticals fall in the category of emerging toxicants and pollutants. These pollutants are currently undergoing a regularization process although the directives and legal frameworks are not set-up yet [13]. Pharmaceuticals find their way into the environment via human and animal excreta from disposal into the sewage system [14]. Their presence in water can also be attributed to pharmaceutical industry waste, hospital waste and therapeutic drugs [15]. They are not only released into the environment after use. Some are disposed during manufacture or as unused or expired drugs [16]. For phytoextraction to function, contaminants must be bioavailable (ready to be absorbed by roots). Bioavailability depends on metal solubility in soil solution. Only metal ions in soil water and in exchangeable positions are readily available for plant uptake. Some metals, such as Zn and Cd, occur primarily in exchangeable form; i.e. readily bioavailable form. Others, such as Pb, occur as precipitates; a considerably less bioavailable form. Therefore, Pb is very difficult to remove. The capacity of a soil to adsorb Pb increases with increasing pH, cation exchange capacity, organic carbon content, soil/water ratio and phosphate levels [17-18]. Plants show some ability to reduce the hazards of organic pollutants [19-21], the greatest progress in phytoremediation has been made with metals [22-24]. Pelargonium grandiflorum is an erect straggling herbaceous shrub, usually growing to a height of 0.75 m. The glaucous stem is soft smooth and shiny. The foliage is attractive, smooth and glaucous. The dull grayish green leaves are deeply palmately lobed, usually 5 cm long and 8 cm wide. The leaf margins are coarsely toothed and some of the leaves have a brownish zonal marking. The fairly large and beautiful flowers vary from creamish-white to pink with darker blotches on the upper two petals. Pelargonium grandiflorum belongs in the family Geraniaceae, a large cosmopolitan family of approximately 11 genera and 800 species in subtropical and temperate regions of the world. The South African genera in the Geraniaceae family are Monsonia, Sarcocaulon, Pelargonium, Erodium and Geranium. There are approximately 270 species of Pelargonium which occur in S, E and NE Africa, Asia, St Helena, Tristan da Cunha, Madagascar, Australia and New Zealand, most of which (± 219 species) occur in southern Africa. Pelargoniums are often wrongly called geraniums [25-27]. Pelargonium grandiflorum will be horticulturally rewarding and easily adaptable to any garden situation. Before planting in the garden plenty of compost to the soil should be added. The plant pelargonium could be as part of a shrubby border or in a large rockery or even around a small pond. Due to being adapted and growing in any condition we select this plant for phytoremediation study.
The aim of the current study is to: (1) Assess the applicability of Plarganium Grandiflorum in removing heavy metals from the contaminated soil after and (2) determine the accumulation of heavy metals in plarganium grandiflorum grown in chemical and toxicology laboratories’ pharmaceutical effluent and wastewater irrigated soil in the vicinity of sewage treatment plant (STP), Pharmacy Faculty, Tehran.
Materials and Method
Effluent samples were collected between 1 April and October 5th, 2015. Sample collection containers (1 L, amber glass) were washed in hot water, rinsed three times with distilled water, rinsed three times with acetone, and then baked in a heated oven at 250ºC for a minimum of four hours. A 24-hour composite sample (500 mL of effluent) was collected by WWTP operators from each WWTP, using their own equipment, and 100 2 mL of a solution containing 5.0 g/L of Na2EDTA and 25 mg/L of ascorbic acid was added at the time of collection. The samples were shipped overnight on wet ice, and stored at 4°C until extraction. Because of the large number of sampling sites and chemical analytes, it was logistically too difficult and expensive to collect and analyze field blanks as well as duplicates from each location. Field blanks were collected from 20% of the sampling sites, with the field blanks being prepared from laboratory distilled water that was transferred into sampling containers and preserved at the time of collection. Duplicates were collected and analyzed for 10% of the sample sites.
Waste water Effluent
Effluents from five educational and research laboratories in pharmaceutical sciences branch, Azad university in Tehran, including, Food Science and Technology research (Effluent 1&2), Toxicology (Effluent 3,4), Analytical chemistry(Effluent 5) were used in this study. Effluent 1 and Effluent 2 were from the same laboratory but were collected on separate occasions with a 3 week time interval. Although Effluent 1 and Effluent 2 come from the same WWTP, they were treated as 2 different effluents due to the variability of their characteristics. This difference is attributed to the significant experiments which occurred following the first sampling event. After collection, the effluent was immediately transported to the research laboratory for analysis. Physico-chemical parameters such as pH, Electrical Conductivity, Total Solids, Total Dissolved Solids, Total hardness, Chloride, Sulphate, Dissolved oxygen, , Calcium, Sodium, Cadmium, Lead, Zinc, Copper, Chrome, Manganese ,Iron and Potassium were analyzed as per the standard methods [28].
The initial concentration of heavy metals/metalloid in the plants and effluents were analyzed before introduction into the studied soil samples. After 10days of treatment up to 60 days in every ten days , final concentration of heavy metals/metalloid in effluent samples and plants were analyzed using Atomic Absorption Spectroscopy . The samples were analyzed by an Atomic Absorption Spectrophotometer Model AA-6200 (Shimadzu, Japan) using an air-acetylene flame for heavy metals and using at least five standard solutions for each metal. All necessary precautions were taken to avoid any possible contamination of the sample as per the AOAC guidelines [29-31]. The efficiency of plant in accumulating heavy metals in their areal parts such as leaf, stem and also root were calculated using bio-concentration factor.
Estimation of Heavy Metals in Effluents
The heavy metals/metalloid in the effluent samples such as Chrome, Zinc, Copper, lead, cadmium, Manganese and Iron were analyzed before and after treatment by AAS after digestion of plant materials by AOAC method [31-32]. The plant samples were washed in deionized water dried (24 hrs at 80°C) immediately to stabilize the tissue and stop enzymatic reactions. After drying, samples were ground to pass a 1.0mm screen using the appropriate Wiley Mill. After grinding, the sample were thoroughly mixed and a 5- to 8-g aliquot withdrawn for analyses and storage [33]. Weighed 0.5 to 1.0 g of dried (80°C) plant material that has been ground (0.5 to 1.0 mm) and thoroughly homogenized and place in a tall-form beaker or digestion tube. Added 5.0 ml concentrated HNO3 (65 %) and cover beaker with watch glass or place a funnel in the mouth of digestion tube and allow to stand overnight or until frothing subsides. Place covered beaker on hot plate or digestion tube into block digester and heat at 125°C for 1 hour. Removed the digestion tube and allowed cooling. Added 1 to 2 ml 30% H2O2 and digest at the same temperature. Repeated heating and 30% H2O2 additions until digest is clear. Add additional HNO3 as needed to maintain a wet digest. After sample digest is clear, removed watch glass and lowered temperature to 80°C. Continued heating until near dryness. Added dilute HNO3 (10%), and deionized water to dissolve digest residue and bring sample to final volume[34].
Total dissolved solids (TDS)
The total solid concentration in waste effluent represents the colloidal form and dissolved species. The probable reason for the fluctuation of value of total solid and subsequent the value of dissolved solids due to content collision of these colloidal particles. The rate of collision of aggregated process is also influenced by PH of these effluents [34].
Chemical oxygen demand (COD)
The chemical oxygen demand test (COD) determines, the oxygen required for chemical oxidation of organic matter with the help of strong chemical oxidant. The COD is a test which is used to measure pollution of domestic and industrial waste. The waste is measure in terms of equality of oxygen required for oxidation of organic matter to produce CO2 and water. It is a fact that all organic compounds with a few exceptions can be oxidizing agents under the acidic condition. COD test is useful in pinpointing toxic condition and presence of biological resistant substances. For COD determination samples were preserved using H2SO4 and processed for COD determination after the entire sampling operation was complete [34-35].
Biochemical oxygen demand (BOD)
For BOD, 5 samples were immediately processed after Collection for the determination of initial oxygen and incubated at 20 °C for 5 days for the determination of BOD5[34-36].
Chlorides
Chlorides are generally present in natural water. The presence of chloride in the natural water can be attributed to dissolution of salts deposits discharged of effluent from chemical industries, oil well operations, sewage discharge of effluent from chemical industries, etc[35].
Sulphates
Sulphate in one of the major cation occurring in natural water. Sulphate being a stable, highly oxidized, soluble form of sulphur and which is generally present in natural surface and ground waters. Sulphate itself has never been a limiting factor in aquatic systems. The normal levels of sulphate are more than adequate to meet plants need [35-38].
Bio concentration Factor
The bio concentration factor is a measure of bioaccumulation of heavy metals. It can be calculated by dividing the trace element concentration in plant tissues (ppm) at harvest by initial concentration of the element in the external nutrient solution (ppm) [ 38-39 ].
BCF = Concentration of the element in plant tissues at harvest (ppm)/ Initial concentration of the element in the external nutrient solution (ppm)
Statistical Analysis
For testing statistical significance, student’s t-test (SPSS-20) was used. Independent sample t-test was used for finding the mean difference of each parameter control with plants.
Results
Chemical extraction of the soil profile before adding specified amounts of heavy metals is shown in the table 1. Data is averages of the effluent profiles.
Table 1: Heavy Metal concentrations in studied effluent Pharmaceutical Laboratories.
| Heavy Metal Content | Concentration (mg/kg DW ± SE*) |
| Lead | 25.894 ± 0.143 |
| Cadmium | 12.340 ± 0.016 |
| Zinc | 26.212 ± 0.111 |
| Chrome | 17.463 ± 0.078 |
| Manganese | 10.212 ± 0.566 |
| Copper | 18.342 ± 0.667 |
| Iron | 43.228± 3.176 |
SE* = Standard Error
The minimum and maximum values of the total solid concentration ranged between 1988-2927 The averaged values ranged between 2044-2356 mg/L for the effluent are well within the maximum permissible limits of 3500 mg/L and according to standards are from U.S. Environmental Protection Agency, the dissolved solids concentration is classified as follows: fresh, 0-1,000 mg/L; slightly saline, 1,000-3,000 mg/L; moderately saline, 3,000-10,000 mg/L; very saline, 10,000-35,000 mg/L; and briny, more than 35,000 mg/L [37-39].
As compared to BOD, COD was very high which is normal for effluent of such pharmaceutical laboratories. The minimum and maximum values ranged between 1039-8022 and the averaged values ranged between 1955-3029 mg/L for the studied effluent. In the present study the values of sulphate for untreated effluent was 720 mg/l which was within the permissible limits of 1000 mg/ L according to the WHO standards [38,39].
This investigation was carried out to determine the accumulation of heavy metals in plarganium grandiflorum grown in chemical and toxicology laboratories’ pharmaceutical effluent and wastewater irrigated soil in the vicinity of sewage treatment plant (STP), Pharmacy Faculty, Tehran. Results in figures 1,2 and 3 showed significant differences in Cr (III) , Cr(VI) and Cd up taking by different parts of plant. The best results for uptake of Nickel, cadmium and chrome was in the soil with pH=6.2 among different samples while for lead up taking was in 6.4 and for zinc up taking was in pH=5.9. This range of pH had no affecting in zinc up taking.
The results revealed that wastewater was highly rich in plant nutrients and heavy metals. The wastewater irrigation significantly (P<0.05/ P<0.01) increased the contents of heavy metals in the soil and plants grown in wastewater irrigated soil. The enrichment of various metals were recorded in the order of Fe >Zn > Pb >Cu > Cd > Cr > Mn in these plants. Moreover contents of different heavy metals in the different parts of plants such as root, young and old leaves and stems showed significant (P<0.05) and positive correlation with contents of Cd (r = +82 to r = +96), Cr (r = +74 to r = +94), Cu (r = +84 to r = +98), Fe (r = +88 to = +98), Mn ( r = +80 to r = +96), Pb (r = +74 to r = +96) and Zn (r = +88 to r = +98) in the wastewater irrigated soil. Although, the contents of Cd, Cu, Fe, Mn, Pb and Zn in soil after growing of plarganium grandiflorum after 60 days were recorded within the prescribed limit of WHO/FAO standards.
At the end of the test period (60 days ) , the plants were taken out of the soil and separated into their main parts; stems, leaves and roots. Each part of the plant was dried and the moisture content was determined. The contaminant was extracted from each part of the plant by using wet digestion method. Lead content in leaves and root in comparison of contaminated soil by pharmaceutical effluent is shown in figure 4.
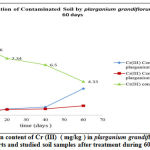 |
Figure 1: The mean content of Cr (III) ( mg/kg ) in plarganium grandiflorum leaves and root parts and studied soil samples after treatment during 60 days. |
Pelargonium (Grandiflorum) has shown ability to extract lead, Cr (III) , Cr(VI) and Cd from contaminated soils; about 35.9 %, 41.9% , 41.7% and 29.8% respectively in the root and leaves zone.
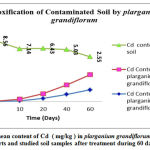 |
Figure 3: The mean content of Cd ( mg/kg ) in plarganium grandiflorum leaves and root parts and studied soil samples after treatment during 60 days. |
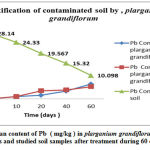 |
Figure 4: The mean content of Pb ( mg/kg ) in plarganium grandiflorum leaves and root parts and studied soil samples after treatment during 60 days. |
Discussion
Kavitha et al. studied the physicochemical analysis of pharmaceutical industrial effluent and treatment plant’s efficiency and found the variation in wastewater characteristics from the inlet point to the outlet point of septic tanks [40]. They observed reduction in the following parameters: TSS 4,300–94 mg/l, TDS 2,846–1,308 mg/l, COD 7,280–9.9 mg/l, BOD 4,132–6.6 mg/l, chlorides 1,000–300 mg/l, sulphates 500–300 mg/l and pH between 7.43 and 7.14. Das et al. studied the control of pharmaceutical effluent parameters through bioremediation [41] . They collected the samples from nine different points situated in the industry and observed the range of sulphates 44–1,527 mg/l, TDS 484–1,452 mg/l, total suspended solids 24–84 mg/l and COD 1,257.9–1,542.9 mg/l. Madukasi et al. characterized the pharmaceutical wastewater and observed the concentration in mg/l for total suspended solids 425 ± 2.3, total dissolved solids 1,600 ± 1.1, total nitrogen 533.7, BOD 146.7 ± 0.3, Zn 0.056, iron 2.1, Mn 0.605, Cu 0.022, acetic acid 422.7, propionic acid 201.3 and butyric acid 304.5 [42]. A suspended growth photobioreactor employing the wild strain of purple nonsulphur photosynthetic bacterium Rhodobacter sphaeroides was utilized to treat the wastewater. The strain was found to be effective in ameliorating hazardous pollutants found in wastewater with over 80 % COD reduction. The strain shows the potential to improve the treatment process and may also be harvested and find use as SCP after further investigation [42].
Gome and Upadhyay utilized ozone for treatment of pharmaceutical wastewater, which required 32.73 mg/l ozone under acidic condition, whereas under alkaline conditions 30 mg/l ozone was needed [43]. They reported that ozonation can improve biodegradability of wastewater at alkaline pH and higher treatment time favoured the enhanced biodegradability of wastewater. Farhadi et al. used electrocoagulation, photoelectrocoagulation, peroxi-electrocoagulation and peroxi-photoelectrocoagulation processes for the removal of COD from pharmaceutical wastewater originating from Osvah Pharmaceutical Company [44]. To´th et al. used distillation and membrane filtration process for treatment of PIWW, which contains high chemical oxygen demand and adsorbable organically bound halogens (AOX) [45]. The distillation was capable of reducing volatile chemical oxygen demand (VOC-COD) and AOX, while the membrane filtration process was beneficial for the treatment of the bottom product of rectification to concentrate the non-volatile pollutants, reducing the COD values close to the emission limits[45]. In 1988 Mayabhate et al. studied and reported the physicochemical and biological treatment of pharmaceutical wastewater [46]. For physicochemical study, they used ferrous sulphate, ferric chloride and alum as coagulants and for biological treatment activated sludge process was used in an oxidation ditch. Sirtori et al. in 2009, reported that pharmaceutical industrial wastewater contained nalidixic acid (an antibiotic pertaining to the quinolone group), which cannot be easily biodegraded [47]. The biodegradability of nalidixic acid was achieved by the chemical oxidation process followed by biological treatment. Chemical oxidation (photoFenton) enhances the biodegradability, followed by biological treatment using immobilized biomass reactor (IBR). The combined efficiency of treatment was over 95 %, of which 33 % corresponded to the solar photochemical process and 62 % to the biological process.
Phytoremediation of wastewater is an emerging low-cost technique for removal of hazardous metal ions from industrial wastewater and is still in an experimental stage. Heavy metals such as cadmium and lead are not easily absorbed by microorganisms. In such case, phytoremediation proves a better treatment tool for bio-treatment because natural plants or transgenic plants are able to bioaccumulate these toxins [48]. Aquatic plants have excellent capacity to reduce the level of toxic metals, BOD and total solids from the wastewater. Billore et al. in 2001 carried out the treatment of industrial effluent with the help of plants Typha latipholia and Phragmitis karka [49]. This treatment eventually led to COD, BOD, total solids and phosphorus content reduction[49]. Some researchers also reported the phytoremediation of phenol from industrial wastewater by peroxidases of tomato hairy root cultures [50-51]. (Gonza´lez et al. 2006).
Conclusion
The present investigation shows that the plarganium grandiflorum plant is effective and inexpensive adsorbent for the removal of Pb, Cd, Cr(III), and Cr (VI) from contaminated soil by heavy metals. Pelargonium grandiflorum will be horticulturally rewarding in every garden. Pelargoniums are a versatile group of plants and are easily adaptable to any garden situation. Planting this pelargonium as part of a shrubby border or in a large rockery or even around every small pond. It could be use to line pathways or as an edging along the front of our flower bed that contains taller shrubs. Light pruning after flowering will neaten the bush and not only enjoy its beauty but also it could be used for treating contaminated soil and rescue the environment . This research conduct adsorption of heavy metals by its leaves and root and proved that this friendly method should gain more attention and research interest for the removal of heavy metals from contaminated soil due to its surface area, adsorption capacity and plenty abundant in nature must be followed seriously. Also, this research suggests more investigations by other genera and families of cost-effective plants.
To quantify the occurrence and the distribution of heavy metals and to prevent them from passing through wastewater collection and treatment systems into soil and ground water bodies represents an urgent task for applied environmental sciences in the coming years. Public acceptance of green technologies is generally higher than that of industrial processes. The responsible organizations should stimulate research to upgrade existing waste water treatment by implementing phytoremediation modules and demonstrating their reliability to the public.
Acknowledgment
Pharmaceutical Sciences Branch, Islamic Azad University (IAUPS) is gratefully acknowledged.
Conflicts of Interest
None of the authors have any conflicts of interest associated with this study.
References
- Swedish Medical Product Agency. Report in Swedish, to the Swedish Government: Miljop¨ averkan fr ˚ an l ˚ akemedel samt kosmetiska och hygieniska produkter. 2004. Available at : http://www.lakemedelsverket .
- A˚ gerstrand, M., Ruden, C. Evaluation of the accuracy and consistency of the Swedish ´ Environmental Classification and Information System for pharmaceuticals. Sci Total Environ., 2010; 408 :2327–39.
- A˚ gerstrand, M., Wester, M., Ruden, C. The Swedish Environmental Classification and ´ Information System for Pharmaceuticals—An empirical investigation of the motivations, intentions and expectations underlying its development and implementation. Environ Internat., 2009; 35:778–86.
- The Appropriateness of Existing Methodologies to Assess the Potential Risks Associated with Engineered and Adventitious Products. Brussels, Belgium.2005.
- Marlene, Å., Linnéa, E., Rud Én, C. Bad Reporting or Bad Science? Systematic Data Evaluation as a Means to Improve the Use of Peer-Reviewed Studies in Risk Assessments of Chemicals, Human and Ecological Risk Assessment: An International Journal, 2014; 20(6): 1427-45. DOI: 10.1080/10807039.2013.854139.
- Handy, R.D., Owen, R., Valsami-Jones, E. The ecotoxicology of nanoparticles and nanomaterials: Current status, knowledge gaps, challenges, and future needs. Ecotoxicol., 2008; 17:315–25.
- Allanou, R., Hansen, B.G,, van der, Bilt, Y. Public Availability of Data on EU High Production Volume Chemicals. 2003. Part 1. Available at http://publications.jrc.ec.europa.eu.
- EDF (Environmental Defense Fund). Toxic Ignorence. The Continuing Absence of Basic Health Testing for Top-Selling Chemicals in the United States. Washington, DC, USA. 1997.
- The Use Of Alternative to Testing on Animals for the REACH Regulation. Helsinki, Finland. 2011.
- Medicinal products. Final Report prepared for Executive Agency for Health and Consumers. 2013. http://ec.europa.eu/health/files/environment/study_environment.pdf .
- Kuster, A., Bachmann, J., Brandt, U. Regulatory demands on data quality for the environmental risk assessment of pharmaceuticals. Regulat Toxicol Pharmacol ., 2009; 55:276–80.
- Ågerstrand, M., Berg,C., Björlenius,B., Breitholtz,M., Brunström, B., Fick,, Gunnarsson,L., Joakim Larsson, D.G,, Sumpter, J.P., Tysklind,M., Rudén, C. Improving Environmental Risk Assessment of Human Pharmaceuticals . Environmental Science & Technology. 2015. DOI: 10.1021/acs.est.5b00302. Available in site: pubs.acs.org/est .
- OECD Guidelines for the Testing of chemicals. Dapnia magna, Reproduction Test. OECD 211., Paris, France. 2008.
- Kümmerer, K. The presence of pharmaceuticals in the environment due to human use–present knowledge and future challenges. J Environ Manage., 2009; 90: 2354-66.
- Daughton, C.G,, Ternes, T.A. Pharmaceuticals and personal care products in the environment: agents of subtle change? Environ Health Perspect .,1999; 107 Suppl 6: 907-38.
- Kümmerer, K. Drugs in the environment: emission of drugs, diagnostic aids and disinfectants into wastewater by hospitals in relation to other sources–a review. , 2001; 45: 957-69.
- Ziarati, P., Determination of contaminants in some Iranian popular herbal medicines. Environ. Anal. Toxicol., 2012; 2: 1. DOI: 10.4172/2161- 0525.1000120.
- United States Environmental Protection Agency., 1992.
- Abdullah, S., Sarem, S. M. The Potential of Chrysanthemum and Pelargonium for Phytoextraction of Lead – Contaminated Soils. Jordan Journal of Civil Engineering., 4, 2010;4.
- Ziarati, P., Alaedini, S. The Phytoremidiation Technique for Cleaning Up Contaminated Soil by Amaranthus SP. J Environ Anal Toxicol. 2014;4: 208. Doi: 10.4172/2161-0525.1000208.
- Boxall, A., Roger, B. Pharmaceuticals and Personal Care Products in the Environment: Regulatory Drivers and Research Needs. QSAR and Combinational Science. 2003; 22: 399-409.
- Ziarati, P., Zolfaghari, M., Azadi, B. The effect of tea residue in promoting phytoremediation of Lavandula angustifoli Int. J. Plant Anim. Environ. Sci., 2014; 4: 479-86.
- Ziarati, P., Ziarati, N.N. , Nazeri, , Saber-Germi, M. Phytoextraction of heavy metals by two Sorghum spices in treated soil using black tea residue for cleaning-up the contaminated soil. Orient. J. Chem., 2015; 31: 317-26.
- Ziarati, P., Iranzad-Asl, , Asgarpanah, J. Companion pelargonium roseum and rosmarinus officinalis in cleaning up contaminated soil by phytoextraction technique the role of companion plants in boosting phytoremediation potential. Int. J. Plant Anim. Environ. Sci., 2014; 4: 424-30.
- South African National Biodiversity Institute’s plant information website: http://www.plantzafrica.com.
- Ahmadi,M., Ziarati ,P., Manshadi,M., Asgarpanah, J., Mousavi, Z . The Phytoremidiation Technique for Cleaning Up Contaminated Soil by Geranium (pelargonium roseum) . Intl J Farm & Alli Sci. 2013; 2 (15): 477-81,
- Manshadi,M., Ziarati, P., Ahmadi,M., Fekri, K . Greenhouse Study of Cadmium and Lead phytoextraction by five Pelargonium spices. Intl J Farm & Alli Sci. 2013; 2 (18): 665-9.
- Ziarati, P., Kermanshah, A., Moslehishad, M. Adsorption Heavy Metal from Contaminated Water by Modified Shell of Wild Endemic Almonds: Amygdalus lycioides and Amygdalus wendelboi. BIOSCIENCES BIOTECHNOLOGY RESEARCH ASIA, 2015; 12(3): 2451-7.
- Standard methods for the examination of water and waste water.22th Edn., APHA,AWWA,WPCF, Washington .D.C.USA. 2012.
- Ziarati, P., Khoshhal, Z. , Asgarpanah , J., Qomi, M. Contaminations of heavy metals in tea leaves, finished tea products and liqour in Gilan province, Iran. I J. Farm Allied Sci., 2013; 2: 383-7.
- Makki, F.M. , Ziarati, P. Determination of histamine and heavy metal concentrations in tomato pastes and fresh tomato (Solanum lycopersicum) in Iran. Biotechnol. Res. Asia, 2014;11: 537-44.
- 1998. AOAC (Association of Official Analytical Chemists). Wet digestion for non –volatile metals in: AOAC official methods of analysis 1998 , 16th edition, 4th revision, vol.1,chapter 9.
- APHA, Standard method for examination of water and waste water (15th Edition), APHA, AWWA, Washington DC. 1995.
- Fu, W, Franco, A., Trapp, S., “Methods for estimating the bioconcentration factor of ionizable organic chemicals”,Environ Toxicol Chem., 2009; 28: 1372-9.
- FAO .1985. Water quality for Agriculture, 1985: Recommendations of the FAO (Food and Agriculture Organization of the United Nations) for the quality of water used for irrigation purposes. http://www.fao.org/DOCREP/003/T0234E/T0234E00.htm.
- World Health Organization, Technical Report Series 778, WHO, Geneva, Switzerland, 1989.
- WHO .1989. Health Guidelines for Use of Wastewater in Agriculture and Aquaculture.
- Jones, J. B., Case V. W.,Sampling, handling, and analyzing plant tissue samples – Soil testing and plant analysis, SSSA, Inc., Madison, WI, 1990.
- Zayed, A., Gowthaman, S., Terry, N., “Phytoaccumulation of trace elements by wetland plants: I.aestivum,” Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 1998; 537: 29-41.
- Kavitha, G.V., Beebi, S.K. Biodegradation of phenol in a packed bed reactor using peat media. Asian J Microbiol, Biotechnol Environ Sci., 2003; 5(2):157–9.
- Das, M.P., Bashwant, M., Kumar, K., Das, J. Control of pharmaceutical effluent parameters through bioremediation. J Chem Pharm Res .,2012; 4(2):1061–5.
- Madukasi, E.I., Daim X,, Hem C., Zhou, J. Potentials of phototrophic bacteria in treating pharmaceutical wastewater. Int J Environ Sci Technol .,2010; 7(1):165–74.
- Gome, A., Upadhyay, K. Biodegradability assessment of pharmaceutical wastewater treated by ozone. Int Res J Environ Sci., 2013; 2(4):21–5.
- Farhadi, S., Aminzadeh, B., Torabian, A., Khatibikamal, V., Fard, M.A. Comparison of COD removal from pharmaceutical wastewater by electrocoagulation, photoelectrocoagulation, peroxi-electrocoagulation and peroxi-photoelectrocoagulation processes. J Hazard Mater.,2012; 219- 220:35–42.
- To´th, A.J., Gergely, F., Mizsey, P. Physicochemical treatment of pharmaceutical process wastewater: distillation and membrane processes. Chem Eng., 2011;55(2):59–67.
- Mayabhate, S.P., Gupta, S.K,, Joshi, S.G. Biological treatment of pharmaceutical Wastewater. Water Air Soil Pollut .,1988; 38(1–2): 189–97.
- Sirtori, C., Zapata, A., Oller ,I., Gernjak, W., Aguera, A., Malato, S. Decontamination industrial pharmaceutical wastewater by combining solar photo-Fenton and biological treatment. Water Res., 2009; 43:661–8.
- Amin, A., Naik, A.T.R,, Azharm M,, Nayak, H. Bioremediation of different waste waters—a review. Cont J Fish Aquat Sci., 2013; 7(2):7–17.
- Billore, S.K., Singh, N., Ram, H.K., Sharma, J.K., Singh, V.P., Nelson, R.M., Dass, P. Treatment of a molasses based distillery effluent in a constructed wetland in central India. Water Sci Technol .,2001; 44(11–12):441–8.
- Gonza´lez, P.S., Capozucca, C.E., Tigier, H.A., Milrad, S.R., Agostini, E. Phytoremediation of phenol from wastewater, by peroxidases of tomato hairy root cultures. Enzyme Microbial Technol., 2006; 39(4):647–53.
- Singh Rana, R., Singh, P., Kandari, V. , Singh, R., Dobhal, R. ,Gupta, S. A review on characterization and bioremediation of pharmaceutical industries’ wastewater: an Indian perspective. Appl Water Sci., 2014. DOI 10.1007/s13201-014-0225-3. Available on site: http://download.springer.com/static/pdf/482/art%253A10.1007%252Fs13201-014-0225-3.pdf.








