Sergei Aleksandrovich Gavrilov1, Alexey Alexeevich Dronov1, Evgeny Pavlovich Kitsyuk2, Egor Aleksandrovich Lebedev 1and Igor Makarovich Terashkevich1
1National Research University of Electronic Technology (MIET) 124498, Moscow, Zelenograd, Shokin Square, 1 2Scientific Manufacturing Complex “Technological Centre” 124498, Moscow, Zelenograd, Shokin Square, 1
DOI : https://dx.doi.org/10.13005/bpj/820
Abstract
The study demonstrates the possibility of the increase of specific capacity of supercapacitors by means of the formation of ultrathin layers of metal oxides (Al2O3, TiO2, NiO) on a surface of an electrode material, which is an oriented arrays of carbon nanotubes. The study discusses the possibility of the application of ultrathin insulating layers of Al2O3 formed by means of ALD method. Deposition of ultrathin oxide layers was carried out by means of successive ionic layer adsorption and reaction (SILAR) and atomic layer deposition (ALD) methods from a gas phase. The authors developed and studied the features of methodologies of conformal deposition of ultrathin layers of metal oxides on complex surface of CNT (carbon nanotubes) based electrode. The study revealed the relationship of the specific capacity of an electrode materials and the thickness of formed layers of metal oxides on its surface. In order to do that the authors prepared experimental specimens, which consisted of two current collectors, made from stainless steel; on the specimen of those collectors composite electrodes, which comprised a separator, made from porous polypropylene, and electrolyte, were synthesized. The specific capacity of a capacitor structure was calculated in the course of the analysis of experimental charge-discharge characteristics. The analysis of the results showed that presence of an ultrathin layer of a metal oxide on a surface of an electrode material based on CNT significantly increases the specific capacity of a structure; however, an optimal thickness of the layer must be specified depending on a material and a method of deposition case-by-case. Presence of an optimum value of thickness of an oxide layer is related to the fact that, from one point of view, the increase of the thickness of a deposited layer increases the influence of pseudocapacity; from another point of view, it can lead to the decrease of the effective area of an electrode surface due to filling of mezapores' space between CNT. It was observed that the optimal method for application of ultrathin oxide layers in mass-production condition is ALD method with implementation of equipment with group loading of slices.
Keywords
atomic layer deposition; SILAR; (transition) metal oxides; supercapacitor; carbon nanotubes; electrode material; nanocomposite; energy accumulation (storage)
Download this article as:| Copy the following to cite this article: Gavrilov S. A, Dronov A. A, Kitsyuk E. P, Lebedev E. A, Terashkevich I. M. Ultrathin Metal Oxides Layer on A Carbon Nanotube Oriented Arrays Surface Formation Process Development and Study for Supercapacitors Electrode Specific Capacity Increasing. Biomed Pharmacol J 2015;8(2) |
| Copy the following to cite this URL: Gavrilov S. A, Dronov A. A, Kitsyuk E. P, Lebedev E. A, Terashkevich I. M. Ultrathin Metal Oxides Layer on A Carbon Nanotube Oriented Arrays Surface Formation Process Development and Study for Supercapacitors Electrode Specific Capacity Increasing. Biomed Pharmacol J 2015;8(2). Available from: http://biomedpharmajournal.org/?p=3825 |
Introduction
One of the most important directions of the development of technologies is the development of efficient and environmentally safe power sources. That kind of devices allows to increase the autonomy of various electric systems from electric cars and electric transport to microsized implanted devices. Electrochemical capacitors with large specific characteristics are promising candidates for a number of applications, because they possess an unique combination of electrophysical characteristics – considerably larger specific capacity, as compared with regular capacitors, and high specific power as compared with accumulators. However, the main problem for researchers in that direction is the increase of capacity of electrochemical capacitors.
Conway (1991) divided electrochemical capacitors of large specific capacity into electric double-layer capacitors (EDLC) and supercapacitors, or pseudocapacitors, which are based on Faraday’s pseudocapacity. That classification is based on fundamental differences of mechanisms of accumulation of electric charge: in EDLC due to formation of double electric layer at transition zone electrolyte-electrolyte; in supercapacitors due to occurrence of reversible oxidation-reduction reaction or electric sorption in thin surface layer of electrode material.
Thus, two main research directions can be specified: firstly, creation of electrodes with high specific surface, and, secondly, development of approaches for the increase of the effect of Faraday’s pseudocapacity. In order to solve the first problem in the last decade all possible modifications of carbon, such as activated carbons, black carbons, graphene and carbon nanotubes, are actively developed and studied (Stoller et al., 2011; Wang et al., 2005; Wang et al., 2012). These materials are cheap and have excellent mechanical, chemical and electrophysical properties, as well as good cyclic stability and long service-life. In order to increase the values of specific capacity and density of energy researches stated to actively developed oxides of transition metals (RuO2, MnO2, NiO, Co3O4, SnO2, ZnO, TiO2, V2O5, CuO, Fe2O, WO3, etc.) as alternative electrode metals (Zhi et al., 2013; Zhao et al., 2009; Amatucci et al., 2001; Pell and Conway, 2004; Zhang et al., 2009).
The presented paper describes the methodology for the development of composite electrode material of supercapacitors based on carbon nanotubes and oxides of transition metals, which, from one point of view, have significant specific surface, and, from another point of view, considerable pseudocapacity. The technology of manufacturing of this kind of materials is based on the application of SILAR and ALD methods from the gas phase on a surface of oriented massifs of carbon nanotubes. Nowadays in MIET ALD method is actively developed and applied; the method is aimed at manufacturing of the antireflection layer of Al2O3, ZnO for solar panels, which allow to increase efficiency of a photoelectric transducer and establish mass-production with implementation of equipment using group loading of slices (Tuzovskii et al., 2015). Further studies in that direction also will allow to use ALD method in the manufacturing technology of planar supercapacitors of increased capacity for very large scale IC and SoC.
Experimental part
Synthesis of carbon nanotubes
Vertically-oriented multi-wall carbon nanotubes arrays were synthesized directly on a surface of current collectors of supercapacitors by means of the method of plasma-enhanced chemical vapor deposition using Oxford PlasmaLabSystem 100 (Nanofab 800 Agile) device according to the methodology described in the previous studies (Tuzovskii et al., 2015). Firstly, a layer of TiN of 25 nm thickness was deposited on substrates by means of the method of magnetron sputtering; the layer acted as diffusion barrier; also a thin layer of Ni with thickness from 5 to 8 nm was deposited using the same method; that layer was used for the formation of nanoclusters of catalyst as the result of thermal treatment of specimens at 280°С temperature in oxygen atmosphere, which was followed by annealing at 450°С temperature in hydrogen atmosphere. With the increase of the thickness of Ni layer the density of oriented arrays of CNT is decreasing. Synthesis of CNT was carried out in mixture of gases C2H2, H2 and NH3 with the working pressure of 2 Torr. Additional treatment with plasm allowed to decrease the process temperature to 500°С. The temperature of the substrate holder was controlled using the thermal sensor. Speed of growth of CNT massif was, approximately, 600 nm per minute.
Layer-by-layer Deposition
One of the methodologies of manufacturing of the composite electrode materials of supercapacitor base on CNT arrays was ALD method; according to that method thin layers of Ti and Al oxides were deposited on a surface of carbon nanotubes. For the formation of functional groups on carbon’s surface, which play a role of active centers for further ALD, we carried out preliminary modification CNT in plasma of N2O gas. High-frequency plasma charge due to development of free active radicals allows to provide hydrophilic properties of carbon’s surface and uniformity of the produced coating. Functionalization at Oxford Plasmalab 100 device was carried out at power of 100 W, gas pressure of 400 mTorr, temperature of substrate of 40°С and time of processing was 30 s.
Further ALD processes were carried using Picosun R150 devices with individual loading of substrates. For deposition of Al oxide we used Al(CH3)3(TMA) and water. Deposition parameters: temperature of working chamber is 300°С, pressure in working chamber is 1.5 mbar, flow of gas-carrier in TMA line is 1 cm3/min, flow of gas-carrier in H2O line is 100 cm3/min. Flow of N in reaction chamber was 300 cm3/min. The deposition consisted from cycles of repeated stages: feeding of TMA into the chamber using gas-carrier, blowing off of the chamber with N, feeding of vapors of deionized water into the chamber, blowing off of the chamber with N. The mentioned stages form one reaction cycle of ALD process for Al oxide, the scheme of which is presented in Figure 1. Speed of deposition of Al oxide film was 0.0097 nm/cycle. The number of ALD cycles was selected on the basis of the required thickness of the oxide layer.
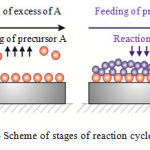 |
Figure 1: Scheme of stages of reaction cycle of ALD process |
For deposition of Ti oxide we used Ti(OH5C2)4(EtTi) and water. Deposition parameters: temperature of working chamber is 300°С, temperature of EtTi source is 145°С, pressure in working chamber is 1.5 mbar, flow of gas-carrier in EtTi line is 150 cm3/min, flow of gas-carrier in H2O line is 100 cm3/min. Flow of N in reaction chamber was 300 cm3/min. The deposition also consisted from cycles of repeated stages: feeding of EtTi into the chamber using gas-carrier, blowing off of the chamber with N, feeding of vapors of deionized water into the chamber, blowing off of the chamber with N. Speed of deposition of Ti oxide film was 0.033 nm/cycle.
The composite electrodes, which were manufactured using layer-by-layer deposition method, were formed on current collectors made from stainless steel film of round shape with 25 mm diameter.
The second method for manufacturing of a composite electrode material of supercapacitors is SILAR (Lindroos et al., 1995). That method was used for deposition of Ni oxide on a surface of the carbon material. Prior to that massifs of CNT were processed in Ar-O2 plasma with incident power of 50 W during 120 s using NIITM MVU Plasma RIT-T device.
As the cation precursor we used 0.01 М Ni(NO3)2·6H2O and liquid ammonia, which was added to the solution in order to create alkaline media (pH=12). As the anion precursor we used water at the temperature of 90 °C. Ni ions were absorbed on the surface of the substrate after it was submerged into the cation solution at room temperature for 30 seconds. After that the substrate was transported to water bath at the temperature of 90 °C for additional 30 s. At that Ni oxide was formed. At the final stage of the cycle the substrate was submerged in deionized water for 30 s for removal of not binded ions of Ni hydroxide. The mentioned stages form one reaction cycle of SILAR process, the scheme of which is presented in Figure 2. The necessary number of deposition cycles was carried out to obtain the coating of a required thickness.
After deposition the specimen were annealed in air at the temperature of 250°C during 2 hours in order to decompose Ni hydroxide, which is formed together with the oxide during the deposition.
The composite electrodes, which were manufactured using layer-by-layer deposition method, were formed on current collectors made from stainless steel film of rectangular shape with dimensions of 20×25 mm2.
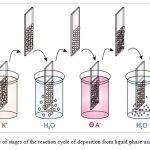 |
Figure 2: Scheme of stages of the reaction cycle of deposition from liquid phase using SILAR method |
Assembly of specimens and measurement of electrophysical characteristics
The study of the influence of the parameters of the composite material comprising CNT and metal oxide on the electrophysical properties of supercapacitors, which are based on them, was carried out on the basis of their charge-discharge characteristics. In order to do that the authors prepared experimental specimens, which consisted of two current collectors, made from stainless steel; on the specimen of those collectors composite electrodes, which comprised a separator, made from porous polypropylene, and electrolyte, made from water solution of sulphuric acid, were synthesized.
Measurement of charge-discharge characteristics was carried out using Elins P-30J potentiometer. Current of charge and discharge varied from 0.5 to 2 mA, and range of voltages was from 0 to 2 V.
Morphology and composition of CNT arrays and composite materials, which are based on them, was controlled by means of SEM method using Agilent 8500 FE-SEM. Control of composition of composite material was carried out by means of the method of EDX analysis.
Results and Discussion
It is known that there are virtually no active groups (except surface defects) on a surface of carbon materials, including carbon nanotubes. Those materials are hydrophobic and are inert to the majoring known reagent used in ALD process (Knez et al., 2007; Lee et al., 2008; Liu et al., 2009), which results in nonunifromity of the obtained coating. The same problem appears in the case of deposition of oxide layers using SILAR method (Lokhande et al., 2011; Lee et al., 2009; Lin et al., 2010), which is based on the application of liquid-phase precursors.
For the formation of functional groups on carbon’s surface, which play a role of active centers for further ALD, we carried out preliminary modification CNT in plasma of N2O gas (Markeev et al., 2013).
In order to form functional groups on a surface of CNT for further deposition by means of SILAR method the authors used the method of surface treatment in plasma of gas mix of Ar and O, which is responsible for physical and chemical mechanisms of activation (Cho et al., 2007; Yang et al., 2014; Hong et al., 2013). For the optimization of modes of treatment we carried out a series of experiments, which included the following stages:
- A surface of carbon nanotubes arrays was subjected to plasma processing in gas mixture of Ar and O2 with various concentration ratios, incident power and length of the process.
- After that layers of NiO were deposited on the surface of CNT arrays, which were processed using SILAR method described above. At that, deposition conditions were always the same.
- After that we carried out studies of morphology of the surface of the formed composites CNT-NiO by means of SEM method.
In the course of the study it was established that plasma pre-processing of CNT arrays increases wettability of surface and conformality of deposition of NiO layers; also, optimum values of power (50 W) and length of the process (120 s) were selected. The further decrease of incident power leads to considerable ion sputtering of CNT.
As the result of studies of the composite material of electrode based on dense CNT arrays by means of SEM method, it was established that deposition of Ni oxide layer took place for the depth of, approximately, 1 µm. In order to increase the depth of the deposition of NiO on CNT arrays by means of SILAR method we synthesized dense CNT arrays with distance between tubes of, approximately, 70-90 nm. Thus, we obtained the depth of deposition up to 3-5 µm. The limitation for further depth of arrays was effective depth of preliminary functionalization of a surface of a carbon material in plasma. That range was selected on the basis of results of study of the composite material by means of SEM method and evaluation of depth of deposition of Ni oxide by means of SILAR method.
Figure 3 presents SEM images of clear CNT, arrays covered with nanoparticles, and nanotubes, which are completely covered by thin layer of Ni oxide.
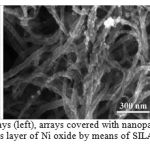 |
Figure 3: SEM images of CNT arrays (left), arrays covered with nanoparticles (center) and arrays covered by continuous layer of Ni oxide by means of SILAR (method) |
It is clear that the modes of treatment in plasma affect the morphology and structure of deposited layers. In the central image there are CNT, covered by Ni oxide in form of independent particles with sizes less than 10 nm. On the right side there are arrays, which are completely and uniformly covered by thin layers of Ni oxide. The increase of the number of SILAR cycles allows to increase the thickness of deposited coatings, which is proved by the increase of CNT diameter.
The similar results were obtained during the study of the process of ALD deposition of metal oxides on a surface of CNT. For the study we selected Ti and Al oxides. The results of SEM analysis showed that without preliminary functionalization of nanotubes in N2O plasma oxides are deposited in a form of separate particles. The thickness of deposited metal oxides varied from 2.5 nm (in that case there was a free space between independent CNT) to 18 nm (all space between CNT was filled by the oxide).
For the study of electrophysical characteristics we prepared experimental specimens of super capacitors based on CNT and composite electrodes (5, 15 and 20 cycles of SILAR deposition of Ni oxide and a series of specimens based on CNT-metal oxide material, which is manufactured by means of ALD method (see Table 1)). During the study we measured charge-discharge characteristics, and after that calculated the values of capacity, which are presented in Table 1.
Table 1: Specific capacity characteristics of supercapacitors with electrodes on the basis of composite CNT/metal oxide
| No. | Method of metal oxide deposition | Electrode structure | Capacity, mF/cm2 |
| 1. | ALD | “Clean” CNT | 0.86 |
| 2. | CNT + 2.5 nm TiO2 | 8.33 | |
| 3. | CNT + 2.5 nm Al2O3 | 4.06 | |
| 4. | CNT + 6.6 nm TiO2 | 3.53 | |
| 5. | CNT + 18.0 nm Al18O3 | 1.65 | |
| 6. | CNT + 18.0 nm TiO18 | 2.25 | |
| 7. | SILAR | “Clean” CNT | 1.60 |
| 8. | CNT + 3.8 nm NiO | 2.16 | |
| 9. | CNT + 11.3 nm NiO | 3.80 | |
| 10. | CNT + 15.0 nm NiO | 4.24 |
It was established that there is a significant increase of capacity in the case of implementation of thin layers of Ti oxide and Al oxide. The increase of the thickness of oxide layer leads to the gradual decrease of values, which can be related to the decrease of effective area of surface due to filling of space between independent CNT.
Figure 4 presents the relationship between the capacity of supercapacitors and the number of SILAR and ALD cycles.
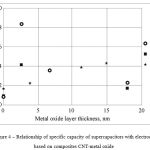 |
Figure 4: Relationship of specific capacity of supercapacitors with electrode based on composites CNT-metal oxide |
From the obtained relationships it can be seen that, in the case of deposition of Al and Ti oxides by means of ALD method, the specific capacity of the composite material of an electrode rapidly increases already at the thickness of the oxide of 2.5 nm, and it has extremum in that area. Rapid increase, is, probably, related to the effect of pseudocapacity of oxide of in the structure of the electrode material. The further decrease of specific capacity can be related to gradual filling of space between CNT by deposited material, which leads to the increase of efficient surface area of the capacitor’s electrode. The maximum obtained values of specific capacity of the experimental specimens were 8.33 and 4.06 mF/cm2 for the composites CNT + 2.5 TiO2 and CNT + 2.5 nm Al2O3, respectively, which in several times exceeds value of specific capacity for “clean” CNT.
In the case of deposition of Ni oxide on a surface of CNT arrays by means of SILAR method we observed linear relationship of capacity of supercapacitors and thickness of the deposited layer. That kind of relationship can be explained by the fact that, in contrast to ALD method, for first cycles of deposition there is no formation of a continuous layer, and only localized zones of the deposited material are formed, which is aggravated by high specific surface of the substrate. The increase of the number of cycles of deposition leads to the increase of the area of those zones, and, finally, they form a continuous ultrathin film. Less dynamic increase of specific capacity can be related to lower deposition of CNT arrays as compared with those used in the experiments with ALD, as well as with discontinuity of the deposited film in the case of small number of deposition cycles.
The experimental specimen No. 10 showed the maximum capacity, and it exceeds the values of the initial clean CNT in 2.5 times. The measured thickness of Ni oxide layer for that specimen was 15 nm, and the calculated speed of deposition was 0.75 nm/cycle.
Figure 5 demonstrates characteristic charge-discharge curves for clean CNT (blue line) and composite electrodes CNT-NiO (black line).
In the first case the curve shows “classical” for EDLC characteristics of charge and discharge – rapid decrease of voltage with time. In the second case there is a peak at 1.5 V voltage, which is more characteristic for accumulators and batteries, which speaks about presence of pseudocapacity of those experimental layers.
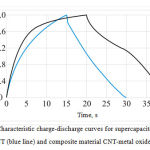 |
Figure 5: Characteristic charge-discharge curves for supercapacitors based on “clean” CNT (blue line) and composite material CNT-metal oxide (black line) |
Conclusion
In the presented study the methods of the increase of capacity of supercapacitors based on CNT by means of deposition of thin layer of metal oxides on a surface are discussed. The study also discusses the influence of preliminary functionalization of CNT on the structure of the deposited layers of metal oxides. It was established that the increase of the thickness of the deposited layer leads to the linear increase of capacity, the value of which for maximum thickness of Ni oxide layer (after 20 cycles of SILAR) was 2.5 times higher as compared to “clean” CNT.
Application of ALD of Ti oxide thin layers on a surface of CNT arrays allowed to increase the capacitance of obtained structure on the order and obtain maximum values of specific capacity.
The obvious advantage of SILAR method is its simplicity and inexpensiveness. However, speed of deposition of the material remains quite low, and time of one deposition cycle can reach tens of hundreds of seconds. Despite of the fact that the speed of ALD from gas phase is also equal to, approximately, one molecular layer per cycle, the method allows to carry out deposition of a material for the whole depth of CNT arrays independently of their density, as well as to more accurately control the thickness of a deposited material. The advantage of ALD process is the conformality of Al2O3 and TiO2 layer deposition on a complex surface with ultralow thicknesses of layers.
In further studies it is planned to carry out experiments and optimization of process of deposition of Ni oxide by means of ALD method (Lindahl et al., 2009; Lu et al., 2008; Jeong et al., 2014), because the latter is of big interest for application as an active electrode material of supercapacitors due to high theoretical specific capacity and comparatively low electric conductivity. Moreover, ALD is the standard method of deposition of materials in micro- and nanoelectronics, which is, undoubtedly, and advantage for development of manufacturing technology of planar supercapacitors as part of very large scale IC and SoC. Industrial application of that technological operation for manufacturing of solid-state electronics products based on implementation of ALD equipment with group loading of substrates, which are developed according to the research program “Research and development of ALD technology of nano-thickness film materials on surfaces of solar panels with the aim to increase generation characteristics”, in the long term will allow to provide mass production of IC and other functional electronic products with higher technical level.
The presented paper is based on the results of the applied research carried out on the basis of Contract No. 14.578.21.0019 between MIET and the Ministry of Education and Science of the Russian Federation. Unique identification number of applied research works – REMEFI57814X0019.
References
- Amatucci, G.G., Badway, F., Pasquier, A.D., and Zheng, T. (2001). An asymmetric hybrid nonaqueous energy storage cell. Soc., 148, A930–A93.
- Cho, H.J., Lee, N., Jang, I., Uh, H.S. and Hong, J.P. (2007). Field Emission from Carbon Nanotubes Etched by a dc Plasma. Journal of the Korean Physical Society, vol. 50, no. 6, p. 1848.
- Conway B.E. (1991). Transition from “Supercapacitor” to “Battery” behavior in electrochemical energy storage. Soc., Vol.138., No.6., pp. 1539-1548.
- Yang, L., Shi, Z. and Yang, W. (2014). Enhanced capacitive deionization of lead ions using air-plasma treated carbon nanotube electrode. Surface and Coatings Technology, vol. 251, pp. 122-127.
- Jeong, M-G., Park, E.J., Jeong, B., Kim, D.H., Kim, Y.D. (2014). Toluene combustion over NiO nanoparticles on mesoporous SiO2 prepared by atomic layer deposition. Chemical Engineering Journal [Internet]. Elsevier BV, 237, 62–9.
- Hong, H.P., Jung, K.H., Kim, J.H., Kwon, K.H., Lee, C.J., Yun, K.N. and Min, N.K. (2013). Percolated pore networks of oxygen plasma-activated multi-walled carbon nanotubes for fast response, high sensitivity capacitive humidity sensors. Nanotechnology, vol. 24, no. 8, p. 085501.
- Knez, M., Nielsch, K. and Niinistö. L. (2007). Synthesis and Surface Engineering of Complex Nanostructures by Atomic Layer Deposition. Advanced Materials 19, no. 21, 3425–3438.
- Lee B., Park S-Y., Kim H-C., Cho K., Vogel E.M., Kim M.J., et al. (2008). Conformal Al2O3 dielectric layer deposited by atomic layer deposition for graphene-based nanoelectronics. Applied Physics Letters, 92(20), 203102.
- Lee, S.W., Kim, B.-S., Chen, S., Shao-Horn, Y. and Hammond, P.T. (2009). Layer-by-Layer Assembly of All Carbon Nanotube Ultrathin Films for Electrochemical Applications. Journal of the American Chemical Society, vol. 131, no. 2, pp. 671-679.
- Liu, C., Wang, C.-C., Kei, C.-C., Hsueh, Y.-C. and Perng, T.-P. (2009). Atomic Layer Deposition of Platinum Nanoparticles on Carbon Nanotubes for Application in Proton-Exchange Membrane Fuel Cells. Small, vol. 5, no. 13, pp. 1535-1538.
- Lin, P., She, Q., Hong, B., Liu, X., Shi, Y., Shi, Z., Zheng, M. and Dong, Q. (2010). The Nickel Oxide/CNT Composites with High Capacitance for Supercapacitor. Soc., vol. 157, no. 7, p. A818.
- Lindroos, S., Kanniainen, T., Leskelä, M., Rauhala, E. (1995). Deposition of manganese-doped zinc sulfide thin films by the successive ionic layer adsorption and reaction (SILAR) method. Thin Solid Films [Internet]. Elsevier BV, 263(1), 79–84.
- Lindahl, E., Ottosson, M., Carlsson, J-O. (2009). Atomic Layer Deposition of NiO by the Ni(thd) 2 /H 2 O Precursor Combination. Chem Vap Deposition [Internet]. Wiley-Blackwell, 15(7-9),186–91.
- Lokhande, C.D., Dubal, D.P. and Joo O.-S. (2011). Metal oxide thin film based supercapacitors. Current Applied Physics, vol. 11, no. 3, pp. 255-270.
- Lu, H.L., Scarel, G., Li, X.L., Fanciulli, M. (2008). Thin MnO and NiO films grown using atomic layer deposition from ethylcyclopentadienyl type of precursors. Journal of Crystal Growth [Internet]. Elsevier BV, 310(24), 5464-8.
- Markeev, A.M., Chernikova, A.G., Chouprik, A.A., Zaitsev, S.A., Ovchinnikov, D.V., Holger, A., Dofler, S. (2013). Atomic layer deposition of Al2O3 and AlxTi1-xOy thin films on N2O pretreated carbon material. Journal of Vacuum Science and Technology A. V. 31(1). P. 01A135-01A135-5.
- Pell, W.G. and Conway, B.E. (2004). Peculiarities and requirements of asymmetric capacitor devices based on combination of capacitor and battery-type electrodes. Power Sources, 136, 334–345.
- Stoller, M.D., Magnuson, C.W., Zhu, Y.W., Murali, S., Suk, J.W., Piner, R. and Ruoff, R.S. (2011). Interfacial capacitance of single layer graphene. Energy Environ. Sci., 4, 4685–4689, DOI: 10.1039/C1EE02322E.
- Tuzovskii, V.K., Gavrilov, S.A., Terashkevich I.M. (2015). Solar cells with a ZnO:Al passivation layer grown by atomic layer deposition. Inorganic Materials. Vol. 51, No. 11, pp. 1205-1207.
- Wang, G.X., Zhang, B.L., Yu, Z.L. and Qu, M.Z. (2005). Manganese Oxide/MWNTs Composite Electrodes for Supercapacitors. Solid State Ionics, 176, 1169–1174.
- Wang, Guoping, Zhang Lei, and Zhang Jiujun. (2012). A Review of Electrode Materials for Electrochemical Supercapacitors. Chem. Soc. Rev. 41, no. 2, 797-828.
- Zhao, X., Chu, B.T.Т., Ballesteros, B., Wang, W., Johnston, C., Sykesand, J.M., and Grant, P.S. (2009). Spray deposition of steam treated and functionalized single-walled and multi-walled carbon nanotube films for supercapacitors. Nanotechnology, 20, (9 pp), doi:10.1088/0957-4484/20/6/065605.
- Zhang, Y., Sun, X., Pan, L., Li, H., Sun, Z., Sun, C. and Tay, B.K. (2009). Carbon nanotube–zinc oxide electrode and gel polymer electrolyte for electrochemical supercapacitors. Alloys Compd., 480, L17–L19.
- Zhi, M. et al. (2013). Nanostructured carbon-metal oxide composite electrodes for supercapacitors: a review. Nanoscale, 5., pp. 72-88








