Natalia Y. Chesnokova, Lyudmila V. Levochkina, Alla A. Kuznetsova and Roza A. Zakharyan
School of Biomedicine, Far Eastern Federal University, 690000, Russia, Vladivostok, Russky Island, Ajax St. 10
DOI : https://dx.doi.org/10.13005/bpj/815
Abstract
The article describes the possibilities of intensifying the extraction of the anthocyanin pigment from black currant in the presence of polysaccharides, as well as the possibility of its use in the production of sweet dishes. The conducted studies show that anionic polysaccharides such as kappa-carrageenan, carboxymethyl cellulose and sodium alginate have the greatest extracting properties. The maximal release of anthocyanin pigment is observed in the reaction medium in the presence of the kappa-carrageenan anionic polysaccharide at a concentration of 0.1 wt.%. Determination of the dynamic viscosity of anionic polysaccharides solutions showed that under the presence of anthocyanin pigments the dynamic viscosity value increases. The dynamic viscosity of the solution of chitosan cationic polysaccharide in the presence of anthocyanin pigments decreases. It is shown that the anthocyanin pigment has antioxidant properties. Adding an 8% solution of anthocyanin pigments and carboxymethyl cellulose (0.05 wt.%) into the production of vanilla cream allows to obtain a product with high organoleptic characteristics.
Keywords
anthocyanin pigment; carrageenan; chitosan; starch; carboxymethyl cellulose; sodium alginate; antioxidant activity; vanilla cream
Download this article as:| Copy the following to cite this article: Chesnokova N. Y, Levochkina L. V, Kuznetsova A. A, Zakharyan R. A. The Use of Anthocyanins of Black Currant and Polysaccharides in the Production of Sweet Dishes. Biomed Pharmacol J 2015;8(2) |
| Copy the following to cite this URL: Chesnokova N. Y, Levochkina L. V, Kuznetsova A. A, Zakharyan R. A. The Use of Anthocyanins of Black Currant and Polysaccharides in the Production of Sweet Dishes. Biomed Pharmacol J 2015;8(2). Available from: http://biomedpharmajournal.org/?p=3817 |
Introduction
Along with the taste properties of products, their appearance and colour are also the key indicators of quality. Colouring of food products is widespread in the culinary practice and must comply with the specifications and technical documentation. Different food dyes that relate to food additives are used to preserve, enhance or impart a specific colour to food products.
Most natural dyes have colour instability during storage and heating and change under the influence of external factors. Consequently, cheaper synthetic dyes ousted natural pigments from food production.
The synthetic dyes currently used for colouring foods often contain harmful or potentially harmful to human health substances. Therefore, recently an issue has been brought up that relates to safety and limiting the use of synthetic dyes in food production and using mostly dyes of natural origin for this purpose. Unlike many synthetic dyes, natural dyes are non-toxic and impart a natural colour. Many of them have a high level of antioxidant activity (Nems et al., 2015; Flanigan, Niemeyer, 2014; Chiou, 2014; Denev et. al.,2010).
There is a global trend of higher demand for red anthocyanin pigments as aside from colouring components they contain other useful biologically active substances. Vitamins, glycosides, organic acids, aromatic substances, microelements contained in anthocyanins have many useful properties: they lower cholesterol, prevent blood clots, increase the elasticity of blood vessels, accelerate wound healing, have a positive effect on vision, contribute to cancer prevention (Lule, Xia, 2005; Konczak, Zhang, 2004; Mokeev, 200; Nakaishi et. al., 2000).
The source of natural red anthocyanin pigments is natural plant materials (flower petals, berries, fruits and vegetables, etc.), as well as wastes of juice and canning industries.
The presence of anthocyanin pigments gives the plants a wide range of shades from red to blue and purple (Gizir et. al., 2008). Anthocyanins belong to the group of flavonoid natural dyes and contain from three to six hydroxyl groups that can be methylated (Tanichev, 1980).
Despite the simplicity of releasing anthocyanin pigments, improvement and development of new methods for their extraction is relevant due to their instability during storage. The literary sources name the production methods of anthocyanins pigment with which the pigment is extracted from plant materials with the extractants of different chemical nature (Jampani, Raghavarao, 2015; Liu et al., 2014; Perevertkina et al., 2011; Churilina et al., 2010; Deineka et al., 2007). Due to the above mentioned, the goal of the present research is to examine the extraction intensification of anthocyanin pigments from black currant in the presence of polysaccharides, as well as the possibility of using this dye in the production of sweet dishes. Selection of polysaccharides as the extracting agents required for anthocyanin pigments release is due to their ability to interact electrostatically and form stable complexes at the account of charged groups present in them (Kruif, Tuinier, 2001). In addition, natural polysaccharides are non-toxic and allow using the system of the anthocyanin pigment-polysaccharide in food production by regulating their colour and consistency.
Research Materials and Methods
Frozen black currant berries were used as objects for the anthocyanin pigments release (Ríbes nígrum). Extraction was carried out with the aqueous polysaccharide solution. The following extractants were used: anionic polysaccharides, kappa-carrageenan (MCS, Korea), carboxymethyl cellulose (MCS, Korea), sodium alginate (Foodchem, China), the cationic polysaccharide chitosan (OOO “Biopolymers”, Russia) and neutral starch polysaccharide (potato starch, Germany). The concentration of polysaccharides in the mixture with water was 0.05, 0.1, 0.2, 0.3, 0.4, 0.5 and 0.6 wt.%.
The crushed raw materials had been treated with the aqueous polysaccharide solution at 70°C for 1 hour and then had been filtered. The intensity of the solution colouring was determined by the change in optical density with the “Unico 1210” spectrophotometer (Russia) at a wavelength of 550 nm.
Determination of the solutions dynamic viscosity was performed on the Brookfield Viscometer DV-II+PRO, USA.
Anthocyanin pigment solutions for the determination of antioxidant properties are prepared by homogenizing frozen fruits with water and thermostating at 70°C and 25°C for 1 hour. Measurement of the antioxidant activity was carried out on the “TsvetYauza-01-AA” device, Russia.
Results of the Research and Discussion
Since the type of a polysaccharide could greatly affect the degree of pigment release from black currant, in this work we studied the dependence of solution colouring intensity on the type of an introduced polysaccharide.
The dependence of the optical density of the anthocyanin pigment from black currant on the concentration and type of polysaccharides is shown in the following Figure 1.
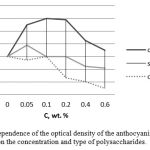 |
Figure 1: Dependence of the optical density of the anthocyanin pigment on the concentration and type of polysaccharides. |
The obtained results show that it is evident that the introduction of the kappa carrageenan anionic polysaccharide into the system as an extractant promotes the release of the black currant anthocyanin pigment. The maximum concentration of the pigment is achieved when administered into the kappa-carrageenan system, 0.1 wt.%. This is due to the electrostatic interaction of the kappa-carrageenan anionic polysaccharide and cationic anthocyanins. A stable complex is formed due to the interaction, contained in a heterocyclic ring of oxygen anthocyanins that is capable of interacting with the negatively charged sulfate groups of the polysaccharide. Further increase in the concentration of kappa-carrageenan may lead to the saturation of the heterocyclic ring, which leads to a lower degree of anthocyanin pigments release.
The use of the chitosan cationic polysaccharide as an extractant does not affect the degree of anthocyanin pigments extraction. This is consistent with the results of the work (Jing et al., 2011), wherein the chitosan cationic polysaccharide is used to purify and stabilize the anthocyanin pigment. Electrostatic repulsion between like-charged functional groups is observed in the solutions of the cationic polysaccharide and cationic anthocyanin pigment. A further increase in the chitosan concentration leads to its absorption by pectins and proteins complexed with anthocyanins, which causes the destruction of the complex and loss of pigment.
Neutral starch polysaccharide at a concentration of 0.05 wt.% insignificantly enhances the degree of anthocyanin pigment extraction. A further increase in the concentration of starch in the pigment-polysaccharide system leads to a slight decrease in the extraction degree.
Thus, the charge of an extractant has a significant impact on the degree of anthocyanins extraction. Adding the anionic polysaccharide of kappa carrageenan to the system makes it possible to extract the most anthocyanin pigment from the anthocynainin containing raw materials.
Since the stability of anthocyanin pigments significantly depends on the system pH (Sui et. al., 2014; Bicard et. al., 1999), perhaps the reason for such a complex dependence of the extraction degree is determined by examining the results of the changes in the pH of anthocyanin pigments solution in the presence of polysaccharides since the polysaccharides are likely to affect the change in the system pH. Dependence of the pH of the anthocyanins pigment solution on the concentration and type of polysaccharides is shown in Figure 2.
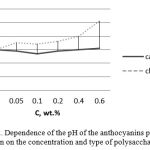 |
Figure 2: Dependence of the pH of the anthocyanins pigment solution on the concentration and type of polysaccharides. |
The obtained data show that the introduction of the anionic polysaccharide of kappa-carrageenan and the cationic polysaccharide of chitosan into the system containing the anthocyanin pigment results in a change in the system pH.
The comparison of the data on the dependence of the colour intensity of the pigment-polysaccharide system (Figure 1) with the change in pH of the anthocyanin pigment solution in the presence of an anionic polysaccharide of kappa carrageenan shows that the smallest value of pH of the solution (pH = 2.8) at a concentration of polysaccharide 0.1 wt.% corresponds to the greatest degree of anthocyanin pigments release.
Introduction of the chitosan cationic polysaccharide system increases the system pH from 3 to 5, thereby reducing the degree of the anthocyanin pigment release. In the acid medium (pH ≤ 2) anthocyanins are resistant to external factors, as they exist in the form of a bright red flavilium cation, so with the given values of pH they are stable (Liu et al., 2014). When pH increases from 2 to 6, colourless pseudo base (carbinol) and chalcone are formed, which leads to the disintegration of anthocyanins and thus to a decrease in the intensity of their colour. This indicates that the decrease in the degree of anthocyanin pigments release occurring together with the increase in the anionic polysaccharide system is due not only to the saturation of the anthocyanins heterocyclic ring, but also depends on the solution pH.
Research on the influence of the anionic polysaccharides of carboxymethyl cellulose and sodium alginate on the extraction degree of anthocyanin pigment is shown in Figure 3. Anionic polysaccharide kappa-carrageenan is used a standard.
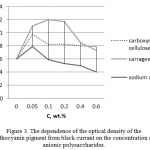 |
Figure 3: The dependence of the optical density of the anthocyanin pigment from black currant on the concentration of anionic polysaccharides. |
As seen from the graphic dependence, the replacement in the kappa-carrageenan system with carboxymethyl cellulose and sodium alginate results in the decrease in their concentrations from 0.1 to 0.05 wt.% for maximal release of the pigment. However, at a given concentration of these polysaccharides in the solution, the optical density values of anthocyanin pigment-polysaccharide solutions are lower than in the presence of kappa-carrageenan and amount to 0.8 and 1, respectively. Perhaps, the nature of the polysaccharides functional groups significantly affects the degree of extraction of anthocyanin pigments. The presence of the most reactive sulfate groups in kappa carrageenan contributes to fuller interaction between kappa carrageenan and anthocyanins (Ermak et al., 2013). Further increase in these polysaccharides in the system leads to a decrease in the degree of extraction of the pigment.
Since polysaccharides in the system act as gelling agents, we studied the dependence of the viscosity of the anthocyanin pigments solution on the type of polysaccharide in this paper.
Figure 4 shows the dynamic viscosity values of solutions of various polysaccharides at a concentration of 0.05 wt.% in the presence of the anthocyanin pigment in the system and without it.
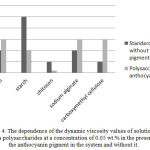 |
Figure 4: The dependence of the dynamic viscosity values of solutions of various polysaccharides at a concentration of 0.05 wt.% in the presence of the anthocyanin pigment in the system and without it. |
The studies have shown that the introduction of the anthocyanin pigment into a system containing a polysaccharide influences the rheological properties of the polysaccharide solution. The system containing the anthocyanin pigment and anionic polysaccharides kappa-carrageenan, carboxymethyl cellulose and sodium alginate had the highest viscosity. In contrast, the presence of anthocyanin pigments and cationic polysaccharide of chitosan or a neutral starch polysaccharide in the system greatly reduces the viscous properties of the solution.
Thus, it has been found that polysaccharides have a noticeable effect on the viscosity of the system containing the anthocyanin pigment causing both its increase and decrease. This change in viscosity is due to the charge of the introduced polysaccharide.
Extending the shelf life of finished products is very important both for producers and for retail outlets. Therefore, in this paper we studied the antioxidant activity of the pigment that affects both the timing of products and their biological value.
The study of the antioxidant properties of anthocyanin pigments extracted from black currant is shown in Figure 5.
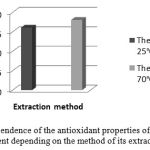 |
Figure 5: The dependence of the antioxidant properties of the anthocyanin pigment depending on the method of its extraction. |
The conducted studies have shown that the solutions containing anthocyanin pigments from black currant have antioxidant activity. The values of antioxidant activity ranged from 16 mg/g to 18 mg/g, depending on the method of release.
The aim to give the product a wide range of colours (from red to violet) and a high antioxidant activity of anthocyanin pigments lead to the possibility of their use in the production of foodstuffs. Application of anthocyanin dyes is very promising for colouring gelled desserts pink and red. Vanilla cream was chosen as an object of colouring. Furthermore, the introduction of the anthocyanin pigment-polysaccharide system into the product at the stage of production allows replacing the gelling agent, incorporated in the recipe.
The examined system consisting of the anthocyanin dye at concentrations of 2, 4, 8, 10 vol.% and carboxymethyl cellulose at a concentration of 0.05 wt.% was added as a stabilizer into vanilla cream during its preparation, replacing gelatine incorporated in the recipe. Depending on the concentration of the used dye, the cream acquired different shades of pink. Vanilla cream with added anthocyanin pigments at a concentration of 8 vol.% had the best organoleptic indicators. At this concentration of the anthocyanin pigment, the cream acquired light pink colour and a pleasant sweet-sour taste of currant. The energy value of vanilla cream with the added anthocyanin pigment amounted to 318.44 kcal.
Conclusions
During the extraction of the anthocyanin pigment, the most effective method is to extract it in the presence of anionic polysaccharides. The charge of the polysaccharide and the pH value of the system are essential during the extraction of the anthocyanin pigment. Furthermore, the use of the anthocyanin dye to impart colour to sweet dishes allows producing a product of pleasant light pink colour, while maintaining the beneficial properties and safety of the product for the consumer.
References
- Bicard V., Faugerousse A., Brouillard R. (1999). Analysis of natural anthocyanins by capillary zone electrophoresis in acidic media. Journal of Liquid Chromatography and Related Technologies, 22, 541-550.
- Chiou A, Panagopoulou E., Gatzali F., De Marchi S., Karathanos V. (2014). Anthocyanins content and antioxidant capacity of Corinthian currants (Vitris Vinifera L. var. Apyrena). Food Chemistry, 146, 157-165.
- Churilina E.V., Korenman Y.I., Sukhanov P.T., Bolotov V.M., Shatalov G.V. (2010). Extraction of natural dyes by hydrophilic polymers. Chemistry of plant raw materials, 2, 153-158.
- Deineka V.I., Khlebnikov V.A., Chulkov L.A., Deineka V.A., Peristiy V.A., Sorokopudov V.N. (2007). Anthocyanins and alkaloids: sorption characteristics by natural clay minerals. Chemistry of plant raw materials, 2, 63-66.
- Denev P., Giz M., Ambrozova G., Lojek A., Yanakieva I., Kratchanova M. (2010). Solid-phase extraction of berries anthocyanins and evaluation of their antioxidative properties. Food Chemistry, 123, 1055-1061.
- Ermak I.M., Reunov A.V., Lapshina L.A., Byankina (Barabanova) A.O., Bratskaya S.Y., Sokolova Y.V. (2013). Physical and chemical and electron microscopic examination of carrageenans – sulfated polysaccharides of red algae and of the Tichocarpaceae and Gigartinaceaeyu families Chemistry of Natural Compounds, 49, 509-511.
- Flanigan P.M., Niemeyer E.D. (2014). Effect of cultivar on phenolic levels, anthocyanin composition, and antioxidant properties in purple basil (Ocimum basilicum L.). Food Chemistry, 164, 518-526.
- Gizir A.M., Turker N., Artuvan E. (2008). Pressurized acidified water extraction of black carrot (Daucuscarota ssp. Satious var. atrorubens Alef.) anthocyanins. European Food Research and Technology, 226, 363-370.
- Jampani C., Rahavarao K. (2015). Process integration for purification and concentration of red cabbage (Brassica oleracea L.) anthocyanins. Separation and Purfication Technology, 141, 10-16.
- Jing P., Ruan S., Dong., Zhang X., Yue J., Kan J., Slavin M., Yu L. (2011). Optimization of purification conditions of radish (Raphanus sativus L.) anthocyanin-rich extracts using chitosan. Food Science and Technology, 44, 2097-2103.
- Konczak L., Zhang W. (2004). Antocyanins – more than nature’s colours, Journal of Biomedicine and Biotechnology, 5, 239-240.
- Kruif C.G., Tuinier R. (2001). Polysaccharide protein interactions. Food Hydrocolloids, 15, 555-563.
- 13. Liu S., Fu Y., Nian S. (2014). Buffering color fluctuation of purple sweet potato anthocyanins to acidity variation by surfactants. Food Chemistry, 162, 16-21.
- Lule S.U., Xia W. (2005). Food phenolics, pros and cons: a review. Food Reviews International, 21(4), 367-388.
- Makeev A.N. (2001). Dyes from natural raw materials for improving the colour and quality of food. Food Ingredients: raw materials and additives, 1, 18-19.
- Nakaishi H., Matsumoto H., Tominaga S., Hirayama M. (2000). Effect of black currant anthocyaniside intake on dark adaptation and VDT work-induced transient refractive alteration in healthy humans. Altern. Med. Rev., 5, 553-562.
- Nems A., Peksa A., Kucharska A., Sokol-Letowska A., Kita A., Drozdz W., Hamouz K. (2015). Anthocyanin and antioxidant activity of snacks with coloured potato. Food Chemistry, 172, 175-182.
- Perevertkina I.V., Volkov A.D., Bolotov V.M. (2011). Effect of glycerol on the extraction of anthocyanin pigments from plant raw materials. Chemistry of plant raw materials, 2, 187-188.
- Sui X., Dong X., Zhou W. (2014). Combined effect of pH and High temperature on the stability and antioxidant capacity of two anthocyanins in aqueous solution. Food Chemistry, 163, 163-170.
- Tanichev S.S. (1980). Anthocyanins in fruits and vegetables. Moscow, p: 302.








