Zabihollah Khaksar* and Hossein Kargar Jahromi
Department of Basic sciences, School of Veterinary Medicine, ShirazUniversity, Shiraz, Iran
DOI : https://dx.doi.org/10.13005/bpj/852
Abstract
Consumption of alcohol during pregnancy causes a wide range of neurophysiological, morphological, and neuropsychological disorders on the central nervous system, especially on the cerebellum of children. Lycopene pigment has neuroprotective effects and antioxidant properties. According to importance of lycopene, this study is aimed to investigate the effect of this pigment on cerebellum histomorphometric alteration of rat pups born to alcohol consuming mother rats. This experimental study was performed on the total number of 32 adult female Wistar rats. According to the purpose of the research the rats were divided into 4 groups of control, lycopene, alcohol, and lycopene and alcohol groups. Each group got pregnant naturally and followed by parturition, the samples were taken from cerebellum of 15- and 30-day-old rat pups after the birth. Various parameters such as thickness of gray/white matter and the number of available cells in the gray and white matters were studied after preparing tissue slides and using Olympus BX51 microscope and Olysia software. The thickness of gray and white matters and the number of available cells of both zones per unit area, in rat pups which their mother just received alcohol (alcohol group) significantly decreased compared with the control group (p>0.05). Compared with alcohol group, the process of decreased thickness of gray and white matter and the number of available cells of both zones per unit area in the group of rat pups which their mother received lycopene and alcohol (lycopene and alcohol group) is less than the control group. Lycopene is able to improve damaging effects of ethanol on the number of cells and thickness of white and gray matter in the cerebellum of rat pups born to alcohol consuming mother rats.
Keywords
Alcohol; Lycopene; Cerebellum; Rat
Download this article as:| Copy the following to cite this article: Khaksar Z, Jahromi H. K. The Effect of Lycopene on Cerebellum Histomorphometric Alteration of Rat Pups Born to Alcohol Consuming Mother Rats. Biomed Pharmacol J 2015;8(2) |
| Copy the following to cite this URL: Khaksar Z, Jahromi H. K. The Effect of Lycopene on Cerebellum Histomorphometric Alteration of Rat Pups Born to Alcohol Consuming Mother Rats. Biomed Pharmacol J 2015;8(2). Available from: http://biomedpharmajournal.org/?p=3686 |
Introduction
Both in rodents and in primates, it is proved that alcohol can be passed from mother to fetus through placenta. Adverse effects of alcohol on intrauterine growth create a wide range of behavioral disorders and neuro-cognitive abnormalities, which are totally called fetal alcohol syndrome (FAS), [1, 2].
The central nervous system is considered as the main target organ for alcohol. The impacts of alcohol on a developing brain is more that an adult brain. Brain different areas including neocortex (particularly frontal lobes), limbic system and cerebellum are very sensitive to the impacts of alcohol [3]. Results of studies show that alcohol reduces proliferation and the number of neurons in the mentioned areas [4]. Alcohol cause neuronal death through increased oxidative reactions and increased expression of inflammatory factors [5].
On the other hand, studies indicate that the range of alcoholic disorders in fetal is associated with defects in motor and balanced coordination which is likely due to created alterations in the natural pathway of cerebellum development [6]. It has been determined in histological studies performed by Ghimire et al. in 2009 on the cerebellum of rat pups born to alcohol consuming mother rats that the diameter of molecular and granular purkinje layers of cerebellum gray matter is intensively reduced [7].
Lycopene is structurally a red carotenoid that mainly is available in tomatoes, watermelon, grapefruit, and apricot [8]. Results of research show that although lycopene does not have the activity of provitamin A, but it can act as an antioxidant and can absorb oxygen radicals. Its antioxidant properties along with reduced oxidative damage to DNA have been proved, in vitro [9]. Among other lycopene properties can mention to neuroprotective [10] and antioxidant properties [12]. It also causes increased cognitive functions [13].
It is known that cerebellum high sensitivity to teratogenic effects of alcohol in germinal period is associated with low levels of endogenous antioxidants in this area of the brain [14, 15]. Therefore, given to neuroprotective and antioxidant effects of lycopene, this study is performed by the purpose of lycopene impacts on the amount of cell population and gray and white matter cerebellum thickness of rat pups born to alcohol consuming mother rats.
Methods: a number of 32 healthy adult female rats with an average weight of 25020 g and with approximate age of 3-3.5 months were prepared in this study. To deal with environmental conditions, the cycle of 12 hours of light and 12 hours of darkness and temperature at 23 ˚C, the rats were kept for a week in the Animal House of Anatomy and Histology Department of Shiraz University of Veterinary Medicine. The rats were then distributed randomly into following groups:
Control group: this group contained 8 conceived rats without any prescription.
Alcohol group: this group contained 8 conceived rats. They received ethanol 20% (diluted with saline 0.9%) by intraperitoneal injection, from two weeks before mating until the sampling date [17].
Lycopene group: this group contained 8 healthy conceived rats. These rats orally received 0.5 ml solution of lycopene with sodium carboxy-methyl-cellulose 0.5% per 100 g of body weight until the sampling date.
Lycopene and alcohol group: this group contained 8 conceived rats. During two weeks before mating, these rats received lycopene and alcohol just like alcohol and lycopene groups with a same dosage and until the sampling date.
Followed by division of rats into different groups, the animals of each group in estrous phase of their sexual cycle were placed in a cage with a male rat for mating and fertilization. Matting was admitted by observation of vaginal plug. After pregnancy and vaginal delivery, all pups born to alcoholic and healthy mothers were kept in the same condition in Animal House. On 15 and 30 days after birth, a number of 6 rat pups of each group were euthanized; their cerebellum removed, and kept in formalin solution (5%, buffered). After passing the process of tissue preparation, paraffin blocks were prepared from the samples using autotechnicon device, and then sections with 5 microns thick were prepared using microtome device and then they were placed on glass slides. The obtained slides were stained green by Hematoxylin and eosin (H&E) and Masson’s Trichrome (MT) staining and covered by lamella at the end. For histomorphometric studies, the obtained sections were examined by optical microscope. The following parameters in cerebellum were measured in all four groups: thickness of gray matter (μ), thickness of white matter (μ), the number of cells available in gray matter (mm2), the number of cells available in white matter (mm2) and the ratio of gray matter to white matter. All above parameters were taken using an Olympus BX51 microscope (made in Japan) and Olysia Software. At least 8 districts in the gray and white matter of each slide were examined and their mean recorded separately, in this assessment. The Student T test and SPSS software were used for data analysis and comparison between experimental and control groups. The significant level was determined at p<0.05.
Findings
According to the results listed in Table 1, the thickness of gray and white matter, and the number of cells per unit area of both regions of the alcohol group of 15-day-old rat pups is significantly reduced compared with the control group (p<0.05). In comparison to alcohol group containing 15-day-old rat pups, reduced process of thickness in gray and white matter and reduced number of cells per unit area of both regions is less in lycopene and alcohol group containing 15-day-old rat pups than the control group.
According to the results listed in Table 2, the thickness of gray and white matter and the number of cells per unit area of both regions in the group of 30-day-old rat pups in alcohol group is reduced compared with the control group, but the decrease is not significant (p<0.05). In comparison to alcohol group containing 30-day-old rat pups, reduced process of thickness in gray and white matter and reduced number of cells per unit area of both regions is less in lycopene and alcohol group containing 30-day-old rat pups than the control group.
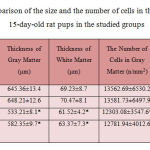 |
Table 1: Comparison of the size and the number of cells in the cerebellum of 15-day-old rat pups in the studied groups |
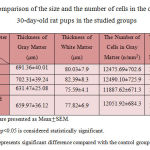 |
Table 2: Comparison of the size and the number of cells in the cerebellum of 30-day-old rat pups in the studied groups |
Ethanol, in this study, caused a significant reduction in the thickness and the number of neurons of gray and white matter in the alcohol groups containing 15-day-old and 30-day-old rat pups. However, reduction in the above parameters in 30-day-old rat pups was not significant compared with the control group. Given to performed studies, alcohol consumption during pregnancy with a time-, age-, and consuming dose-dependent manners will affect on development of fetal brain [19]. In fact, total events and stages of neuronal developments including proliferation, migration, differentiation, synapse formation and myelination can be affected by ethanol consumption [20]. Although neuronal development of all regions of the brain is affected by alcohol, but the cortex and cerebellum neurons are more susceptible to the damaging impacts of ethanol. Detrimental effects of ethanol can be mentioned as follows, neurons death, reduced number of neurons, and impairment in neuronal differentiation [21]. Studies’ results in this regard showed increased oxidative stress and increased induction of cell death by ethanol consumption. Several studies both in vitro and in vivo conditions showed that alcohol consumption during pregnancy increases the level of oxidative stress through an increase in production of free radicals and/or through a creation of impairment in antioxidant defense system and as a result will induce apoptosis and cerebellum neurons death in the rodents brain [22, 23, 24].
The obtained results from this study show that lycopene is able to improve detrimental effects of ethanol on the thickness and the number of neurons in the white and gray matter of the cerebellum in 15- and 30-day-old rat pups born to alcohol consuming mother rats. The antioxidant activity of lycopene in cell culture and animal models has been widely studied and showed that lycopene is able to neutralize free radicals [25]. It is found that lycopene ability to absorb free radicals is two times more than beta-carotene and ten times higher than alpha-tocopherol. There are also experimental evidences regarding the ability of lycopene for turning off oxygen radicals, nitrogen dioxide, thiol, and sulfonyl [26].
Studies indicate that lycopene has neuroprotective effect and is able to prevent nervous system disease caused by destruction and degeneration of neurons, including Alzheimer and Parkinson diseases. A conducted study on cultured rat cortical neurons by Qu et al in 2011 revealed that lycopene has protective effect against neurotoxicity of β-amyloid [27]. β-amyloid is a main pathogenic factors of Alzheimer’s disease. Lebda et al in a study performed in 2011 indicated that lycopene creates protection against environmental neurotoxins and against excessive levels of certain elements such as manganese, through employment of its extremely potent antioxidant properties [28].
Wei et al in 2010 showed that lycopene can prevent the inflammatory response to acute stroke and can reduce domain of damaged area in the brain [29].
Conclusion
It seems that lycopene through inhabitation of oxidative stress and inflammatory responses induced by ethanol can improve toxic and destructive effects of ethanol on cerebellum neurons of rat pups born to alcohol consuming mother rats.
References
- Katzung B. Basic and Clinical Pharmacology. 9th ed ed.Translated by Malek Atayi. Tehran: Nasle Farda; 2004; p. 473
- BG, et al. Maternal risk factors for fetal alcohol syndrome and partial fetal alcohol syndrome in South Africa: a third study. Alcohol Clin Exp Res.2008;32(5):738-53.
- W.J. Chen, S.E. Maier, S.E. Parnell, J.R. West, Alcohol and the developing brain: neuroanatomical studies, Alcohol Res. Health 2003;27 : 174–180.
- Cai L, Bian M, Liu M, Sheng Z, Suo H, Wang Z, “et al”, Ethanol-induced neurodegeneration in NRSF/REST neuronal conditional knockout mice, Fei J,Neuroscience 2011; 181:196-205.
- Crews FT, Nixon K, Mechanisms of neurodegeneration and regeneration in alcoholism, Alcohol Alcohol 2009; 44(2):115-27.
- Guerri C, Bazinet A and. Riley E.P.Foetal Alcohol Spectrum Disorders and Alterations in Brain and Behaviour. Alcohol & Alcoholism2009;44(2).108-114.
- Ghimire S.R, Saxena AK, Rai D,Dhugel S.Effect of maternal alcohol consumption on Cerebellum of rat pups:a histological study.Nepal Med Coll J,2009;11(4):268-71.
- Holic CN, Giovannucci EL, Ronser B, Stampfer MJ, Michaud DS. Prospective study of intake of fruits, vegatables and carotenoids and the risk of adult glioma. Am J Nutr. 2007; 85(3): 864-77.
- Sahlin , E. , Savage , G.P. , Lister , C.E. Investigation of the antioxidant properties of tomatoes after processing. Journal of Food Composition & Analysis. 2004;17: 635-647.
- Hsiao G, Fong TH, Tzu NH, Lin KH, Chou DS, Sheu JR. A potent antioxidant, lycopene, affords neuroprotection against microglia activation and focal cerebral ischemia in rats. In Vivo. 2004;18(3):351-6.
- Gunasekera RS, Sewgobind K, Desai S, Dunn L, Black HS, McKeehan WL, et al. Lycopene and lutein inhibit proliferation in rat prostate carcinoma cells. HNUC. 2007;58(2):171-7.
- Kuhad A, Sethi R, Chopra K. Lycopene attenuates diabetes-associated cognitive decline in rats. Life sciences. 2008;83(3):128-34.
- Akbaraly NT, Faure H, Gourlet V, Favier A, Berr C. Plasma carotenoid levels and cognitive performance in an elderly population: results of the EVA Study. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2007;62(3):308-16.
- LeBel, C.P.; Odunze, I.N.; Adams, J.D., Jr.; Bondy, S.C. Perturbations in cerebral oxygen radical formation and membrane order following vitamin E deficiency. Biochem. Biophys. Res. Commun. 1989, 163, 860–866.
- Abel, E.L.; Hannigan, J.H. Maternal risk factors in fetal alcohol syndrome: Provocative and permissive influences. Neurotoxicol. Teratol. 1995, 17, 445–462.
- Bertrand PC, O’Kusky JR, Innis SM. Maternal dietary (n-3) fatty acid deficiency alters neurogenesis in the embryonic rat brain. The Journal of nutrition. 2006;136(6):1570-5.
- Pina MM, Cunningham CL. Effects of dopamine receptor antagonists on the acquisition of ethanol-induced conditioned place preference in mice. Psychopharmacology. 2014;231(3):459-68.
- Kumar P, Kalonia H, Kumar A. Lycopene modulates nitric oxide pathways against 3-nitropropionic acid-induced neurotoxicity. Life sciences. 2009;85(19):711-8.
- Sakata-Haga H, Sawada K, Hisano S, Fukui Y. Administration schedule for an ethanol-containing diet in pregnancy affects types of offspring brain malformations. Acta Neuropathol 2002;104:305–312.
- Levitt P. 1998. Prenatal effects of drugs of abuse on brain development. Drug Alcohol Depend 51:109–125.
- Kumar A, LaVoie H.A, DiPette D.J and Singh U.S .Ethanol Neurotoxicity in the Developing Cerebellum: Underlying Mechanisms and Implications. Brain Sci. 2013, 3, 941-963.
- Kumar, A.; Singh, C.K.; Lavoie, H.A.; Dipette, D.J.; Singh, U.S. Resveratrol restores Nrf2 level and prevents ethanol-induced toxic effects in the cerebellum of a rodent model of fetal alcohol spectrum disorders. Mol. Pharmacol. 2011, 80, 446–457.
- Smith, A.M.; Zeve, D.R.; Grisel, J.J.; Chen, W.J. Neonatal alcohol exposure increases malondialdehyde (MDA) and glutathione (GSH) levels in the developing cerebellum. Brain Res.Dev. Brain Res. 2005, 160, 231–238.
- Olney, J.W.; Tenkova, T.; Dikranian, K.; Muglia, L.J.; Jermakowicz, W.J.; D’Sa, C.; Roth, K.A. Ethanol-induced caspase-3 activation in the in vivo developing mouse brain. Neurobiol. Dis. 2002, 9, 205–219.
- Cohen LA. A reviw of animal model studies of tomato carotenoids, lycopene and cancer chemoprevention. Exp Biol Med. 2002; 227: 864-8. [PMID:12424327]
- Yaping Z, Suping Q, Wenli Y, Zheng X, Hong S, Side Y, et al. Antioxidant activity of lycopene extracted from tomato paste towards trichloromethyl peroxyl radical CCl3O2. Food Chemistry. 2002; 77: 209-12.
- QU M, Li L,Chen C,Li M, et al.Protective effects of lycopene against amyloid β-induced neurotoxicity in cultured rat cortical neurons.Neurosci Lett.2011;503(3):286-90.
- Lebda MA, El-Neweshy MS, El-Sayed YS. Neurohepatic toxicity of subacute manganese chloride exposure and potential chemoprotective effects of lycopene. Neurotoxicology. 2012 Jan;33(1):98-104. Epub 2011 Dec 16.
- Wei Y, Shen XN, Mai JY, Shen H, Wang RZ, Wu M. The effects of lycopene on reactive oxygen species and anoxic damage in ischemia reperfusion injury in rats. Zhonghua Yu Fang Yi Xue Za Zhi. 2010 Jan;44(1):34-8.







