Rahmanian Elham1, Tavakol Koukhdan Ebrahim2, Kargar Leila*3, Poorgholami Farzad4, Shafiei Jahromi Nazanin5, Pourahmadi Mohammad6 and Kargar Jahromi Hossein6
1Anatomy and Embryology, Cellular and Molecular Gerash research Center, Grash School of Medical Science, Shiraz University of Medical Science, Shiraz, Iran. 2Department of Anatomical Sciences, School of Veterinary Medicine, Shiraz University, Shiraz Iran. 3Department of Radiology, Yasuj University of Medical Science, Yasuj , Iran. 4Reaserch center for non-Communicable Diseases, Jahrom University of Medical Sciences, Jahrom, Iran. 5Department of Nursing, Islamic Azad University, Firuzabad Branch, Firuzabad, Iran. 6Zoonoses Research Center, Jahrom University of Medical Sciences, Jahrom, Iran.
DOI : https://dx.doi.org/10.13005/bpj/851
Abstract
Paraquat is an herbicide with a liquid formulation and dark green color. It is used in lands as a general, contact and leaf-poisoning. Herbicide paraquat “Gramaxon” has high toxicity after preparation and is absorbed through the skin. Malathion is a contact insecticide which is effective on pests through digestive and respiratory systems. Since both materials are abundantly used in agriculture today, and agricultural products are daily used by human, so, the toxins directly and indirectly have adverse influence on different body systems, especially on reproductive system. So, this study is aimed to investigate herbicide paraquat and organophosphate pesticide malathion on changes of LH, FSH, estrogen, and progesterone hormones in female rats. a number of 80 adult female Wistar rats were used in this study. They at first divided into control, blank, experimental paraquat, and experimental malathion groups. Each of the experimental groups were subdivided then into 3 groups of 10 each including, experimental P1 (daily received 1 mg/kg paraquat), experimental P2 (daily received 2 mg/kg paraquat), and experimental P3 (daily received 4 mg/kg paraquat); and experimental M1 (daily received 10 mg/kg malathion), experimental M2 (daily received 20 mg/kg malathion), experimental M3 (daily received 40 mg/kg malathion). Malathion and paraquat toxins were injected using insulin syringe. Variations of LH, FSH, estrogen, and progesterone hormones were measured by ELISA test. hormonal measuring results in groups receiving paraquat indicate that the concentrations of FSH hormone in all experimental groups have significant increase compared with the control group. Progesterone hormone levels in experimental P1 and P2 groups have significant reduction and significant increase compared with the control group, respectively. Obtained results in groups receiving malathion show that LH hormone levels in different groups have no significant change compared with the control group (p<0.05), however FSH hormone levels in these groups have significant increase compared with the control group. Estrogen hormone levels in experimental M1 and M2 groups have significant decrease and there is no significant change in the levels of estrogen hormone in experimental M3 group. Progesterone hormone levels show significant decrease in the experimental groups compared with the control group (p<0.05). the results of this research indicate that herbicide paraquat and organophosphate pesticide malathion by production of free radicals, probably have adverse effects on sex hormones of female rats and oogenesis process.
Keywords
Paraquat; Malathion; Sex Hormones; Rats
Download this article as:| Copy the following to cite this article: Elham R, Ebrahim T. K, Leila K, Farzad P, Nazanin S. J, Mohammad P, Hossein K. J. The Effect of Herbicide Paraquat and Organophosphate Pesticide Malathion on Changes of Sex Hormones in Female Rats. Biomed Pharmacol J 2015;8(2) |
| Copy the following to cite this URL: Elham R, Ebrahim T. K, Leila K, Farzad P, Nazanin S. J, Mohammad P, Hossein K. J. The Effect of Herbicide Paraquat and Organophosphate Pesticide Malathion on Changes of Sex Hormones in Female Rats. Biomed Pharmacol J 2015;8(2). Available from: http://biomedpharmajournal.org/?p=5853 |
Introduction
By production of chemical pesticides, human decides to control own environment to protect agricultural product cultivate for food supply, against pests. The antiquity of chemical opposition with pests and herbal diseases returns to more than a thousand years before Christ1. Paraquat is an herbicide in the category of bipyridylium2. As a powerful herbicide due to unique characteristics, paraquat has remarkable consumption and production in all around the word. Its chemical formula is N, N’-dimethyl-4, 4’-bipyridinium dichloride. Paraquat is mainly used as herbicide and causes serious lung damage and other injuries in mammals. This herbicide is highly toxic for human (especially with delayed toxicity mechanism to human) and animals. This kind of toxicity is
associated with mitochondrial and microsomal oxidation and reduction systems3. The mechanism of paraquat toxicity is often related to production of superoxide anions, which can lead to production of large amounts of reactive oxygen species (ROS), such as hydrogen peroxide and superoxide anions. The two free radicals are considered as two major toxicants. These radicals are highly toxic and are intensively combined with macromolecules and cause serious damages to various organs4, 5. As a strong peripheral toxic compound, paraquat affects on the growth and development of amphibians. Followed by orally absorption, paraquat accumulates in all important body organs, particularly the lungs (more than 10 times higher than plasma) as an optional member and due to the high volume of distribution. So that in case of severe poisoning, death occurs from respiratory failure due to pulmonary edema and fibrosis6. The impact of paraquat and its mechanism in reproductive system of male rats is decreased weight of male reproductive systems (includes testes, epididymis, seminal vesicles, and prostate), as well as reduced number of sperms, cell division, and spermatogonial cells7. Researches performed on bacteria, mice and human revealed that paraquat has no mutagenic and carcinogenic effects. However, it weakens the immune system and endometriosis. Reduced number of sex cells and considerable shrinkage in Bulb/c race of mice is caused by paraquat, as well8. Malathion is introduced in 1950 as the first phosphorous compounds of mammals. Rapid decomposition in body into non-toxic compounds followed by consumption is the important prominence of organophosphorous compounds. Unlike chlorinated organic pesticides, this compound does not accumulate in body tissues of living organisms and biological cycles1. Its scientific name is malathion or carbofos and its trade name is malathion and malathiosol1. Malathion is derived from phosphorodithioic acid1. Its utilization for a certain period may be toxic for some cells such as T cells9. Liver tissue is a candidate for receiving of malathion most effects10. This toxic compound also causes disturbance of DNA in body11, induction of free radicals production and oxidative stress in the brain, and increased activity of anti-oxidant enzymes12. Use of lethal and sub-lethal dose of this poison can also increase fatty acids, glycerol, and lipase activity in tested tissues and this can have a negative impact on reproductive activity and causes decline in the population13. By inhibition of acetylcholinesterase enzyme, steroidogenesis process is inhibited by malathion, too1. Followed by absorption of organophosphorus (OP) pesticides in body, they undergo numerous bio-transformation reactions. Since OP compounds are lipophilic, they permeate to skin readily. The bio-transformation reactions are mainly derived toward formation of polar compounds to achieve the ability of exertion from kidneys. OP compounds in biological terms may convert to some metabolites which their toxicity changes determinately15. Given to the above as well as widespread use of paraquat and malathion and various direct and indirect effects of these toxins on reproductive system, so this study is aimed to investigate the impact of an herbicide (paraquat) and an organophosphate pesticide (malathion) on changes of sex hormones in female rats.
Methods
This study was conducted in vitro and completely random. All ethical use of animals was observed in this study. A total number of 80 adult female Wistar rats weighing 20015 g and at 100- 120 days of age were produced from Razi Vaccine and Serum Research Institute of Shiraz. The rats were then transported to Jahrom and kept for 2 weeks in Animal House of Islamic Azad University under in vitro condition, including 212 ÚC and a cycle of 12 hours of light and 12 hours of darkness. Malathion insecticide and paraquat pesticide were prepared from Moshkfam and Alafkosh Companies, respectively. The toxins were injected intraperitoneally for 14 days. Since malathion and paraquat are soluble in saline, so malathion and paraquat lethal doses were determined 80 mg per kilogram of body weight and 8 mg per kilogram of body weight, respectively. The rats were then divided randomly as follows:
Control
The rats in this group were kept in normal condition and without receiving any medications.
Blank
The rats in this group received saline intraperitoneally. Paraquat receiving groups Experimental P1: the rats in this group intraperitoneally received paraquat at 1 mg/kg body weight. Experimental P2: the rats in this group intraperitoneally received paraquat at 2 mg/kg body weight. Experimental P3: the rats in this group intraperitoneally received paraquat at 4 mg/kg body weight.
Malathion receiving groups
Experimental M1: the rats in this group intraperitoneally received malathion at 10 mg/kg of body weight.
Experimental M2: the rats in this group intraperitoneally received malathion at 20 mg/kg of body weight.
Experimental M3: the rats in this group intraperitoneally received malathion at 40 mg/kg of body weight.
After the injections, all rats were anesthetized and dissected and blood samples were obtained directly from their hearts using insulin syringe (5 cc). Subsequent to separation of blood serum, the concentrations of LH, FSH, estrogen, and progesterone hormones were measured by ELISA method in the laboratory of Jahrom’s Medical Science University. One-way analysis of variance (abbreviated one-way ANOVA) was used for comparison between treatments. P<0.05 was considered as significant level. SPSS version 15 was also used for data analysis and to perform statistical tests.
Results
The obtained results related to changes of sex hormones in paraquat receiving groups are as follows. As listed in Table 1, it is clear that the amounts of LH hormone in all the experimental groups do not show significant changes compared with the control group. However, FSH levels in all groups reveal a significant increase compared with the control group (P<0.05). There is no significant change in estrogen levels in all the experimental P groups. A significant decrease is observed in progesterone hormone levels in the experimental P1 group compared with the control group. However, this amount shows a significant increase in the experimental P2 group compared with the control group and no significant change is observed in the experimental P3 group. The obtained results related to changes of sex hormones in malathion receiving groups are as follows. According to Table 2, LH hormone levels in all the experimental groups do not show a significant change compared with the control group. FSH hormone levels in all groups show a significant increase compared with the control group (p<0.05). Estrogen levels in the experimental M1 and M2 groups show significant decrease compared with the control group (p<0.05). However, this amount in the experimental M3 group do not show a significant change compared with the control group. Progesterone hormone levels show a significant reduction compared with the control group (p<0.05).
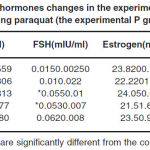 |
Table 1: Sex hormones changes in the experimental groups receiving paraquat (the experimental P groups) |
Discussion
A- Interpretation of results related to changes in sex hormones in the experimental groups receiving paraquat (the experimental P groups) With increasing population in human society, preparation of food supplies is impossible without use of advanced scientific farming. In modern agriculture, people have to use pesticides, herbicides and chemical fertilizers, as well. All of these pesticides, herbicides and chemical fertilizers while serving humans are somehow cause problems. One of the toxins is paraquat which its biochemical parameters and mechanisms have not been identified accurately. Studies have shown that oxidative stress plays an important role in pathogenesis of several diseases such as cancer, diabetes, atherosclerosis, and lung disease and cataracts16. Oxidative stress is caused by free radicals and mitochondria are known as the main location for production of free radicals 17. Mitochondria are targets of choice for paraquat toxicity in animal and herbal tissues18. On the other hand, lipids peroxidation increases adverse effects such as increased stiffness of membrane, reduction of intracellular materials, decreased in fluidity of membrane, and osmotic fragility19. Paraquat also reduces the amounts of glutathione and glucose 6-phosphate dehydrogenase, while increases superoxide glutathione, glutathione peroxidase catalase, and superoxide. This reflects defense and impact on antioxidant system of rats20. Performed studies have shown till now some impacts of paraquat such as histopathological changes in rats21 and ovarian and testicular atrophy 22. Paraquat impacts investigation on different generations of particular race of hen during certain periods revealed that paraquat caused reduced
fertility, and ovaries and fallopian tubes weight loss, as well as impairment in newborn baby chicks23. Other performed studies on paraquat and glyphosate effects on ovaries and testes of amphibians found that paraquat inhibits 17-beta estradiol, resulting impairment in steroidogenesis pathway. This may be caused by oxygenic reagents or reactive oxygen species (ROS)24. So it can be concluded that if an ovarian tissue is damaged,
then it will face with impairment in the secretion of estrogen and progesterone hormones. This impairment appears in the form of decreased or increased levels of the hormones. On this basis, significant reduction of progesterone hormone levels in the experimental P1 group compared with the control is quite logical. In this study, the estrogen
levels increase in the experimental P1 group and decrease in the experimental P2 and P3 groups, however these changes are not significant compared with the control group. Changes of LH and FSH hormones may initially seem to be due to increased amount of GnRH which increases blood serum FSH, but since these changes have no effect
on blood serum levels of LH, they could not be effective in increase of GnRH. FSH levels show significant increase in all the experimental P groups compared with the control group (p<0.05). According to the researches of Hemayatkhah Jahromi et al. in 2008, the concentration of these hormones reduced in adult male rats. This is contrary to what we have achieved in this study which could be due to the released free radicals and lipid peroxidation (1-captopril). This causes tissue changes and as a result impairments in secretion of hormones. This difference also may depend to gender of rats. B- Interpretation of results related to changes in sex hormones in the experimental groups receiving malathion (the experimental M groups) So far, most researches have been done in the terms of the effect of organophosphorous pesticides with various doses on different organs and on different animals and each of which obtained different results. The results obtained inthis research that show malathion effect on ovaries of rats can complete available data about the effects of this pesticide on various species investigated
previous. Organophosphorous pesticides have alkylation properties and thus can affect on nuclear DNA25. Malathion increases malondialdehyde levels in ovarian tissue that may be caused by release of free radicals as a result of body lipid metabolisms. On the other hand, malathion causes ovarian tissue damages and weight loss; with increased doses, malathion toxicity reduces healthy follicles and increases the number of atretic follicles. It also changes corpus luteum26. Other studies show that increased levels of malondialdehyde and lower antioxidant defense system in body can lead to destruction of cells 27. So this conclusion that levels of estrogen and progesterone hormones significantly decreased is quite logical since these two hormones secrete from ovaries and any tissue damages to ovaries impairs the secretion of these hormones. Studies also reveal that malathion does not significantly change levels of FSH and 17-beta estradiol in dairy cows. However, progesterone level was significantly decreased after injection of this pesticide. Malathion induces oxidative stress and production of free radicals and increases the activity of antioxidant enzymes28. Malathion effects on concentrations of acetylcholinesterase (AChE) and 5-delta, 3-beta hydroxysteroid and phosphate dehydrogenase were invstigated in a study. This pesticide induces chromosomal aberrations in somatic cells (bone marrow) and early sexual cells (primary spermatocytes)30, 31. It should be noted that ovarian is the secretion site of estrogen and progesterone hormones, while gonadotropin hormones are released from pituitary gland. There is no research about malathion impact on pituitary tissues. On the other hand, ovarian hormones influence on secretion of pituitary gonadotropin secretion during the estrous cycle. Of course, the main role of hypothalamus should not be ignored.
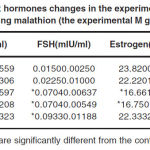 |
Table 2: Sex hormones changes in the experimental groups receiving malathion (the experimental M groups) |
Conclusion
According to this study, it can be told that malathion pesticide and paraquat herbicide can have devastating effects on female rats reproductive indicators and these effects may be extended in lesser extent to human. Of course, in this case the use of pesticides as well as the amount of exposure to it is very important. So care must be taken in generalizing of this research findings to human and possible experiments should be carefully done.
References
- Najafi GH, Salame S, Karimi A. Effects of diazinononadult mousetestisinrats: histopathological study. Medical Journal of Urmia, 20(4): 313- 319 (2010).
- Ghazi Khansari M, Maghsoud SH, Sotoudeh M, Shekiba B, Mohammadi Karkani A, Azar P, Esmaili, Effects of captopril in prevention of pulmonary fibrosis caused by paraquat investigation of Nitric Oxide role, Department of Pharmacology (Pour Sina), 2004
- Taylor NL, Day DA, Millar AH. Environmental stress causes oxidative damage to plant mitochondria leading to inhibition of glycine decarboxylase. J Bio chem. 277(45)42663 (2002).
- Farrington JA, Ebert M, Land EJ, Fletcher K. Bipyridylium salts and related compounds.v. pulse radiolysis studies of the reaction of paraquat radical with oxygen.Implication for the mode of action of bipyridyl herbicides. Biochim Biophys acta; 314(3):372-81 (1973).
- Crouch E. Pathobiology of pulmonary fibrosis. Am J Physiol; 259: 159-84 (1999). 6. Scheffler IE. A century of mitochondrial research: achievements and perspectives. Mitochondrion, 1(1): 3-31 (2000).
- Anuar MD.Zain, the evalution of the toxic effect of paraquat and its mechanism of action on reproductive system of male rats, master of science, 2007, p-um 1107.
- Hemayatkhah Jahromi V, Parivar K, Bahoaldini AA, Kafilzadeh F. The effect of paraquat herbicide on histological changes of testes, fertility, spermatogenesis process, and hormonal pituitary-gonadal axis in Balb/ C race mice. Iranian Journal of Biology, 21(3): 527-535 (2008).
- Rakhshani E, Talebi AA, Taheri AH. Principles oftoxicology agricultural. Publicationsdictionary; 156- 177 (2006). [ text in persian ].
- Guyton AC, Hall JE. Textbook Of Medical Physiology, 2th ed. Pub:Elsevier; 1038- 1025 (2006).
- Brealy CY,Walker GH, Bladwin BC. Esterases activities in relation on the different toxicity of primiphos- metyl to birds and mammals. Pestic sci, 11: 546-554 (1980).
- Rumiza AR, Khairul O, Mohd II, Rajamuhamad Z, Rogayah AH. Determination of malathion levels and the
effect of malathion on the growth of Chrysomya megacephala (Fibricius) in malathion- exposed rat carcass. Trooical biomedicine, 25(3):184- 190 (2008). - Ansiwal KB, et al. Ultra structural changes in clitellum region of Eisenia foetida after treatment with malathion. The biosean, 2: 207-210 (2010).
- Blasial J, Trzeciak A. Single cell gell electrophoresis (comet assay) as a tool for environmental biomonitoring an example of pesticides. Polish journal of environmental studies, 4: 189-194 (1998).
- Gurushankara HP, Meena KD, Krishnamur SV, Vasadev V. Impact of malathion stress on lipid metabolism in Linnonectus limnocharis. Pesticide biochemistry and physiology, 88: 50-56 (2007).
- Scheffler IE. A century of mitochondrial research: achievements and perspectives. Mitochondrion, 1(1): 3-31 (2000).
- Cadnes E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and again. Free radic boil med, 29(3-4): 222-230 (2000).
- Taylor NL, Day DA, Millar AH. Environmental stress causes oxidative damage to plant
mitochondria leading to inhibition of glycine decarboxylase. J Bio chem. 277(45)42663 (2002). - Aydin S, Aral I, Kilic N, Bakan I, Erman F. The level of antioxidant enzymes, plasma vitamins C and Ecement plant workers. Clin chim acta, 341 (1-2): 193-198.
- Sanhita R, Aanab S, Amitabha R. effect of paraquat on antioxidant system in rats. Indian journal of experimental biology, 45: 432- 438 (2007).
- Alex BH. The effect of paraquat on histopathologic changes of rats. Biochem Physiol, 80(3): 53-57 (1996).
- Balford AU, Anderson A.Oncogenicity study of paraquat in rats. Toxiol, 51(3):61-67(1991).
- Cheryl AB. Effect of paraquat on reproduction and growth in northern bobwhite. J wildlife manage, 49(4): 1068- 1073 (1985).
- Luana Q, Ennio M, Oretta M, Massimo B. effect of paraquat and glyphosate on steroidogenesis in gonads of frog Rana esculenta in vitro. Pesticide biochemistry and physiology, 93 (2): 91-95 (2009).
- Dutta HM, Maxwell LB. Diazinon induced endocrine disruption in bluegill, sunfish, Lepomis macrochirus. Ecotoxicology and environmental safety, 60: 21-27 (2003).
- Kos ND, Kayhan FE, Sesal C, Muslu MN. Dose- dependent effect of endosulfan and malathion on adult Wistar albino rat ovaries. Pak j boil sci, 12(6): 498- 503 (2009).
- Marjani AJ. Malondialdehydelevelsin plasma anderythrocyteantioxidantenzyme activity india betic II patients. Journal of ArdabilUniversityof Medical Sciences, 6(2): 183- 187 (2007).
- Fortunato JJ, Feier G, Vitali AM, Petronilho FC, Dal- pizzol F, Quevedo J, Neurochem. Malathion induced oxidative stress in rats brain regions, 31(5): 671-8 (2006).
- Inbaraj RM, Haider S. Effect of malathion and endosulfan on brain acetylcholine esterase and ovarian steroidogenesis of Channa punctatus (bloch). Ecotoxicol environ saf, 16(2): 123-128 (1988).
- Salvadori DMF, Ribeiro LR, Pereira CAB, Becak W. Cytogenetic effect of malathion insecticide on somatic and germ cells of mice. Genetic toxicology, 204(2): 283- 287 (1988)
- Asmarian SH, Rahmanian koshkaki E, Jamali H and et al. Antioxidative Effect of Vitamin C on Toxic Effects of Malathion on Reproductive Tract Physiology in Female Rats. Advances in Environmental Biology 4(2): 1-10 (2014).








