Ebrahim Tavakol Koukhdan* and Zabihollah Khaksar
Department of Anatomical Sciences, School of Veterinary Medicine, Shiraz University, Shiraz Iran.
DOI : https://dx.doi.org/10.13005/bpj/894
Abstract
This study was conducted to evaluate the effects of Fenugreek seed extract (trigonellafoenum-graecum) on development of diabetic rats offspring spinal cord structure, especially in the cervical enlargement. Sixteen healthy female rats were divided into four groups (normal control, diabetic control, control Fenugreek seed and Fenugreek seed treatment). Diabetes was induced in four groups by Streptozotcin(50mg/kg/IP) and mating by male rat and vaginal plaque mentioned as a positive sign of pregnancy. Two groups received extract and the two others were given distilled water. After 20 days rats were scarified and their offspring were removed. Spinal cords were collected, processed and embedded in paraffin and various histological parameters were determined. Results showed that mean body weight of the diabetic control group was significantly higher (P≤ 0/05). Reduction in the vertical and transverse diameters and number of neurons in the diabetic group was significant (P≤ 0/05). Also, mean blood sugar was decreased significantly (p<0.05). Based on the results, it can be concluded that Fenugreek seed extract reduced the blood sugar and diabetes effects in the spinal cord.
Keywords
Fenugreek seed extract -Streptozotocin- Diabetes - Rat - Spinal cord
Download this article as:| Copy the following to cite this article: Koukhdan E. T, Khaksar Z. Study of Fenugreek Seed Extract Effect (Trigonella Foenum-Graecum L) on Histomorphic Structure of Cervical Enlargement of the Spinal Cord Development During Gestational Age of 20 Day Offspring of Diabetic Rats. Biomed Pharmacol J 2015;8(2) |
| Copy the following to cite this URL: Koukhdan E. T, Khaksar Z. Study of Fenugreek Seed Extract Effect (Trigonella Foenum-Graecum L) on Histomorphic Structure of Cervical Enlargement of the Spinal Cord Development During Gestational Age of 20 Day Offspring of Diabetic Rats. Biomed Pharmacol J 2015;8(2). Available from: http://biomedpharmajournal.org/?p=3884 |
Introduction
Diabetes mellitus is one of the most common metabolic diseases of human beings. Mortality and morbidity increase in diabetics mainly because of the associated chronic complications such as nephropathy and atherosclerosis. It is characterized by hyperglycemia together with biochemical alterations of glucose and lipid metabolism (Jensen T et al., 1988).
Pancreas, by producing insulin allows the body to use glucose efficiently. However, with diabetes the pancreas insufficiently controls the insulin, causing blood sugar levels to rise (Jones C.W., 2001). Diabetic pregnancy is seen in over 8% of all pregnancies (Moore T, 2004). In diabetic mothers, during pregnancy placental transport of glucose and other nutrients intensifies due to an increased availability at the maternal site, resulting in their expansion in fetal and neonatal Macrosmia (Persson and Hanson 1998).The elevated serum glucose concentration in mother, accompanied by hyperglycemia in the fetus, leads to degranulation of the fetal β-cells and results in fetal hypoinsulinemia. Indeed, in the majority of newborns of badly controlled diabetic mothers [blood glucose >16.7 mmol 1 44 −1] pancreatic β-cells show degranulation (F. A. Van assche et al. 1985). The risk for diabetes is significantly higher in the offspring of mothers who have non-insulin-dependent diabetes (Knowler et al. 1985). In addition, maternal diabetes increases the risk of hypoglycemia and other chemical imbalances such as low calcium and magnesium levels (Jones C.W., 2001). Data indicate that pre-gestational maternal diabetes is associated with strong teratogenic effects on the kidney, urinary tract, and heart and is also strongly associated with multiple congenital abnormalities (Chung and Myrianthopoulos 1975).The incidence of major malformations in women with type 1 diabetes is about 5%. Fetal anomalies account for almost half of prenatal deaths in diabetic pregnancies. Diabetes is not associated with increased risk for fetal chromosomal abnormalities (Cuningham et al. 2005). One of the mammalian systems that are clearly impaired in diabetes is nervous system. Diabetes leads to numbness at the nerve endings (Cecil et al. 2003). Atherosclerosis in brain is another prominent change in diabetes. Studies have shown that obstruction of feeding vessels of nerves due to diabetes causes nerve bundles death and myelin destruction (Harrison et al. 2000). An increased number of malformations occur in infants born from mothers with maternal diabetes involving the central nervous system (CNS), the spinal column, the ribs, and the urinary tract (Aberg et al. 2002).Specific types of anomalies in CNS which are linked to maternal diabetes are anencephaly, spina bifida, and hydrocephaly (Cuningham et al. 2005). Maternal diabetes induced hyperglycemia and acute intracerebral hyperinsulinism reduces fetal brain neuropeptide Y concentrations (Singh et al. 1997).Usually, fetuses with CNS anomalies or those exposed to adverse conditions which may affect CNS functioning take longer to habituate, or fail to habituate (Hepper and Leader 1996). The organization of behavioral states is poorer in fetuses of diabetic mothers than non-diabetic mothers (Mulder and Visser 1991).
The effect of diabetes on the brain suggests that it may lead to neurophysiological alterations, cognitive abnormalities, changes in both brain function and structure such as white matter hyperintensities, and the gray matter density changes in type I diabetes, which suggests that persistent hyperglycemia and acute severe hypoglycemia have an impact on brain structure (Musen et al., 2006). In addition, white matter microstructure deficits were seen in type I diabetes (Kodl et al., 2008). Type 1 diabetes has had decreased gray matter and white matter in some parts of the cerebrum (Northam et al., 2009).One study demonstrated that hyperglycemia prevents differentiation of cortical neurons and causes oxidative stress in diabetic pregnancies of rat (Guleria et al., 2006). Diabetes induces alteration in the dendritic morphology of cortical neurons (Martínez-Tellez et al., 2005), hippocampal neuronal apoptosis (Li et al., 2002), and disturbs the proliferation and cell death of neural progenitors (Gao and Gao, 2007).
It is known that maternal diabetes can cause changes in the size and number of neurons in the spinal cord. This causes symptoms and irreversible damage on the offspring’s nervous system (Hematian H and Khaksar Z, 2012). Therefore, in pregnancies affected by diabetes there will be a higher risk of the development of type 2 diabetes so medical intervention is necessary (Gabbe SG et al., 2004).
Medicinal plants have been used in traditional medicine for many years and have a special role in the treatment of many diseases. So far, more than 1200 medicinal plants with effects in reducing blood sugar levels or reducing the complications of diabetes are known (Falah Hosseini H et al., 2006).In this study Fenugreek seed extract was selected. Fenugreek seed (Trigonella foenum-graecum) is an annual plant in the family Fabaceae. The hypoglycemic effect of Fenugreek seeds has been studied in many animal model systems as well as in humans, especially in IDDM and NIDDM patients. In one study it was shown that a broken fiber in fenugreek seed extract caused a significant effect on glucose homeostasis in animal models of type I diabetes by delaying carbohydrate digestion and absorption, and enhancing insulin function (Hannan JM. et al. 2007). Feeding fenugreek seed powder for 3 weeks has a protective role against histopathological abnormalities in tissues of rats that have been exposed to alloxan (ShaliniThakran. et al. 2004). It has been proven that, compared with diabetic group, rats treated with Trigonella foenum-graecum extract had an increase in body weight and a decrease in kidney /body weight ratio (p<0.05) and also, compared with diabetic group in a dose-dependent manner, rats treated with Fenugreek seed extract had lower blood glucose, glycated hemoglobin, triglycerides, total cholesterol and higher density lipoprotein-cholesterol (p<0.05) (Wan-Li Xue. et al. 2007).Currently, the main treatment of diabetes mellitus is using insulin and hypoglycemic drugs, but these compounds have numerous side effects. Identification of compounds with minimal side effects (especially in pregnancy) which reduce blood sugar and have no adverse effect on the fetus seems necessary.
Materials and Methods
The purpose of this investigation is to evaluate the possibility of Fenugreek seed extract treatment of congenital spinal cord malformations in offspring of diabetic rats at day 20 compared to the offspring of mothers who did not receive the extract.
Extracts
Fenugreek seeds were collected from a local market in Iran and botanically authenticated with voucher specimens that were deposited in the National Herbarium, Iran. Seeds were ground to fine powder by a cyclotec grinding machine. The powder (1000gr) was extracted with aqueous 96% ethanol, the extracted solution was evaporated, and a dark brown extract was obtained (Eseyin, O. et al., 2007, Pereira, J.A. et al., 2007, Jones C.W., 2001).
Animals
16 Adult male Sprague-Dawley rats weighing 180-220 g aged 3 to 4 months were used throughout this study. All institutional guidelines were adhered during the care and treatment of the animals used in the present study. Animals were maintained on a 12 h light/dark cycle at21 ± 2ᵒC. A standard pellet diet and water were supplied ad libitum. The overall nutrient composition of the diet was36.2% carbohydrate, 20.9% protein, 4.4% fat and 38.5% fiber, with metabolisable energy content of 11.8 MJ/kg(2820 kcal/kg).
Induction of Diabetes
Induction of type I diabetes was performed by a single intraperitoneal injection of overnight fasted rats (180-220g) with 50 mg/kg streptozotocin body weight freshly dissolved in 0.5 M-citrate buffer (pH 4.5) (Wyngaarden JB et al., 1982). The blood glucose level was checked 7 days after streptozotocin administration. Animals having blood glucose levels 300-200mg/dl were considered as diabetic (Eseyin, O et al., 2007).
The selected rats were randomly allocated into 4 groups (A, B, C and D).
Group A: (normal control): Healthy mothers were given normal saline.
Group B: (control Fenugreek seed extract): Healthy mothers were given Fenugreek seed extract at a dose of 1,000 mg / kg daily.
Group C: (diabetic control): Diabetic mothers were given normal saline.
Group D: (Fenugreek seed extract treatment): Diabetic mothers were given Fenugreek seed extract at a dose of 1,000 mg/kg daily.
Sampling
Female rats were caged with males overnight. Females were considered 1 day pregnant on demonstrating vaginal plaque (Kar, A, 2003).
At the end of 20 days, blood glucose was measured and afterward, the animal was sacrificed and offspring removed and weighed. Offspring were fixed in 10% formalin. Abdominal piece of whole offspring was removed and spinal cords collected. The 5-6 μ serial sections were made using paraffin embedding techniques and were stained by hematoxyline-eosin. Factors were measured in 200x magnifications. Histometrical studies were done using Olympus microscope and Olysia software.
Histomorphometry Study
These factors were studied by light microscopy on the cervical enlargement of spinal cord:
- Vertical and transverse diameters of the spinal cord (μ)
- Vertical and transverse diameter of the central canal of the spinal cord (μ)
- The number of cells on gray matter
- The number of cells on white matter
- The volume ratio of gray matter to white matter (G/W)
Statistical Analysis
Spss software version 11.5 was used for statistical analysis. Group’s variance was analyzed by one way ANOVA test for significant differences. P<0.05 was considered statistically significant
Results
The result showed that the STZ (50 mg/kg) induced diabetic mellitus in rats and blood glucose reached to 468 ± 19.322mg/dl. Administration of Fenugreek seed extract (1000mg/kg) reduced the blood glucose significantly so that it reached 123.5 ± 4.342 mg/dl at the end of 20 days (p<0.05). Mean bodyweight of offspring in group C were increased 5/27±0/45gram and group D were decreased 4/30±0/54gram. The weights of offspring in diabetic groups were significant. (Table 1)
Histometrical Change
Table 1 shows the parameters of the cervical enlargement of the spinal cord in four groups. Transverse diameter of the spinal cord on the diabetic control offspring is significantly (P ≤ 0/05) less than the other groups and transverse diameter of the central canal in the diabetic control group was lower, and this difference was significant (P ≤ 0/05).
Vertical diameter of the spinal cord in the diabetic control shows a significant decrease but vertical diameter of the central canal was significantly higher (P ≤ 0/05) (figure 1). The number of cells in the white and gray matters in the diabetic control group was significantly less than other groups (P ≤ 0/05).The volume ratio of gray matter to white matter in the diabetic control group was significant (P ≤ 0/05) (figure 2)
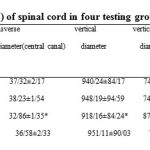 |
Table1: The mean parameters (± S.E) of spinal cord in four testing groups
|
Discussion
Type II diabetes is one of the most common metabolic diseases in the world and its rate is increasing, the most important characteristic of this disease is chronic hyperglycemia (Rahimi R et al., 2005). Medicinal plants have traditionally been used worldwide to control diabetes (Bailey C.J and Day C, 1989, Gray AM andFlattPR, 1997). In this study results showed a significant increase in the body weight of offspring of diabetic mothers (P ≤ 0/05). This increase in offspring weight is known as macrosomia. Macrosomia occurs due to increased transport of glucose and other nutrients from the mother to the fetus via the placenta (Jones CW, 2001). In this situation, many offspring store extra fat in the shoulders and trunk, macrosomia occurs in all organs except the brain (Cuningham FG et al., 2005).
In the cervical enlargement of spinal cord, study of transverse diameter and the vertical diameter of the central canal, showed a significant reduction in the diabetic group (P ≤ 0/05). According to previous studies, the effects of diabetic neuropathy have been reported in various nerves such as the sciatic nerve neuropathy (Artico M et al., 2002) and the autonomic nervous system (Guyton AC and Hall JE, 2006) The neuropathy in this area of the spinal cord can cause the malformation.
This study shows a significant reduction in the number of cells in the gray and white matter in the diabetic group (P ≤ 0/05). Maternal diabetes caused decreased proliferation and increased apoptosis of the neuroepithelial cells in the developing spinal cord of embryos from diabetic mouse (Gao Q and GaoYM, 2007). Fetal macrosomia and infant respiratory distress syndrome, cardiomyopathy, hypoglycemia, hypocalcemia, hypomagnesemia, polycythemia, and hyperviscosity all can occur as a result of maternal hyperglycemia (Jones CW, 2001). Also, due to maternal diabetes blood vessel damage occurs, which results in nerve cells death (Wyngaarden JB and Smite LH, 1982). Decreased proliferation and increased apoptosis were in neural progenitor cells exposed to high glucose, and high glucose-induced apoptosis in neural progenitor cells was associated with activation of caspase-3; so, high glucose disturbs both proliferation and cell death of neural progenitors in the developing spinal neural tube (Gao Q and GaoYM, 2007).This study showed the positive effect of Fenugreek seed extract in the prevention of diabetes effect on the cervical spinal cord of the fetus which may be due to the antioxidants in the extract (Tripathi UN and Chandra D, 2010).
Conclusion
Maternal diabetes reduces fertility and the number of offspring; it causes changes in different parts of spinal cord in laboratory animals like the size and number of neurons. This causes symptoms and irreversible damage to the offspring’s nervous system. According to biochemical and histological findings of previous studies and the results of this study it is concluded that one of the mechanisms of hypoglycemia Fenugreek seed extract is reconstruction and repair of pancreatic islets and subsequent increase in insulin levels. Fenugreek seed extract has proven antioxidative effects (Tripathi UN and Chandra D, 2010), and also has a protective effect deal against the diabetic nephropathy (Xue W et al., 2011).It has been proven that administration of Soluble dietary fiber fraction to normal, type 1 or type 2 diabetic rats significantly improved oral glucose tolerance, and also, total remaining unabsorbed sucrose in the gastrointestinal tract of non-diabetic and type 2 diabetic rats following oral sucrose loading was significantly increased by Fenugreek seed extract.Glucose transport in 3T3-L1 adipocytes and insulin action were increased by Fenugreek seed extract (Hannan JM. et al., 2007). In another study the therapeutic role of Fenugreek seed powder in type-1 diabetes was attributed to the change of glucose and lipid metabolising enzymeactivities to normal values, so it caused glucose homeostasis to stabilize in the liver and kidney (Jayadev R et al., 2001).
Thus, in this study the therapeutic effect of Fenugreek seed extract was demonstrated, and due to the limited side effects it could be a possible new therapeutic treatment in type-1 diabetes.
References
- Aberg A, Westbom L, kallen B (2002) congenital malformation among infants whose mothers had gestational diabetes or pre-existing diabetes. Early Human Development 61: 85-95
- Artico M, Massa R, Cavallotti D (2002) Morphological changes in the sciatic Nerve of Diabetic Rats Treated with low Molecular weight Harparin op 2123/parnaparin. Anat Histol Embryol 31: 193-7
- Bailey CJ, Day C (1989) Traditional plant medicines as treatments for diabetes. J Diabetes Care 12: 553 – 64
- Beischer NA, Wein P, Sheedy MT, Steen B (1996) Identification and treatment of women with hyperglycaemia diagnosed during pregnancy can significantly reduce perinatal mortality rates. Aust NZ J Obstet Gynaecol 36: 239-47
- Christopher T. Kodl, Daniel T. Franc, Jyothi P. Rao, Fiona S. Anderson, William Thomas, Bryon A. Mueller, Kelvin O. Lim and Elizabeth R. Seaquist (2008) Diffusion Tensor Imaging Identifies Deficits in White Matter Microstructure in Subjects with Type 1 Diabetes That Correlate With Reduced Neurocognitive Function. Diabetes 57(11): 3083-3089
- Chung CS, Myrianthopoulos NC (1975) Factors affecting risks of congenital malformations. I. Analysis of epidemiologic factors in congenital malformations. Report from the Collaborative Perinatal Project. Birth Defects Orig Artic Ser 11(10):1-22
- Cuningham FG, Lolo KG, Blome AL, Hat JC (2005) William’s obstetrics.In: 22nd ed. McGraw-Hill: New York, PP 1170-87
- J.H. Mulder, G.H.A. Visser (1991) Growth and motor development in fetuses of women with type-1 diabetes. II. Emergence of specific movement patterns. Early Hum Dev 25(2):107–115
- Elisabeth A. Northam, Debbie Rankins, Ashleigh Lin, R. Mark Wellard, Gaby S. Pell, Sue J. Finch, George A. Werther and Fergus J. Cameron (2009) Central Nervous System Function in Youth With Type 1 Diabetes 12 Years After Disease Onset. Diabetes Care 32(3): 445-450
- Erdemoglu N, Kupeli E, Yesilada E (2003) Anti-inflammatory and antinociceptive assessment of plants used as remedy in Turkish folk medicine. Journal of Ethnopharmacology 89: 123-9
- Eseyin O, Ebong P, Ekpo A, Igboasiyi A, AndOforah E (2007) Hypoglycemic effect of the seedextract of Telfairia occidentalis in rat. Pakistan Journal of Biological Science 10(3): 498-501
- A. VAN ASSCHE, L. AERTS (1985) Long-Term Effect of Diabetes and Pregnancy in the Rat. Diabetes 34: 116-118
- Falah Hosseini H, Fakhrzadeh H, Ardeshir Larijani B, Shikh Samani AH (2006) Review of anti-diabetic medicinal plant used in traditional medicine. Journal of Medicinal Plants 5: 85-60
- Gabbe SG, Gregory RP, Power ML, Williams SB, Schulkin J (2004) Management of diabetes mellitus byobstetrician-gynecologists. Obstet Gynecol 103(6): 1229-34
- Gao Q, Gao YM (2007) Hyperglycemic condition disturbs the proliferation and cell death of neural progenitors in mouse embryonic spinal cord. Int J Dev Neurosci 25(6): 349-57
- Gray AM, Flatt PR (1997) Pancreatic and extrapancreatic of the traditional anti-diabetic plant, Medicago Sativa (Lucerne). Br J Nutr 78: 325 -34
- Greene MF, Hare JW, Cloherty JP, Benacerraf BR, Soeldner JS (1989) First-trimester hemoglobin A and risk formajor malformation and spontaneous abortion in diabetic pregnancy. Teratology 39: 225
- Guyton AC, Hall JE (2006) Textbook of medical physiology. 11th ed. Elsevier Saunders: Philadelphia, PP 961-76
- Hannan JM, Ali L, Rokeya B, Khaleque J, Akhter M, Flatt PR, Abdel-Wahab YH (2007) Soluble dietary fibre fraction of Trigonella foenum-graecum (fenugreek) seed improves glucose homeostasis in animal models of type 1 and type 2 diabetes by delaying carbohydrate digestion and absorption, and enhancing insulin action. Br J Nutr 97(3): 514-21
- Harrison TR, Braunwal DE, Wilson JD (2000) Harrison’s principles of internal medicine. 15th ed. McGraw Hill: New York,PP 2109-42
- Hematian H, Khaksar Z (2010) Evaluation of maternal diabetes effects on Lumbosacral portion of spinal cord in neonate rat by morphometry. Journal of Shahrekord University of Medical Sciences 12(1): 53-9
- Jayadev R, Dhananjay G, Araga R, Rao P, Yadava K, Najma Z. Baquer (2001) Trigonella foenum graecum (fenugreek) seed powder improves glucose homeostasis in alloxan diabetic rat tissues by reversing the altered glycolytic, gluconeogenic and lipogenic enzymes. Mol Cell Biochem 224(1-2):45-51
- Jones CW (2001) Gestational diabetes and its impact on the neonate. Neonatal Network 20(6): 17-23
- Kar A (2003) Comparative evaluation of hypoglycaemic activity of some Indian medicinal plants in alloxan diabetes rats. J Ethnopharmacol 84(1): 105-8
- Khaksar Z, Jelodar GH, Hematian H (2010) Evaluation of maternal diabetes effects on Lumbosacral portion of spinal cord in neonate rat by morphometry. Journal of Shaheed Sadoughi University of Medical Sciences 18(1): 56-63
- Knowler W, Pettitt DJ, KunzelmanCL, Everhart J (1985) Genetic and environment determinants of non-insulin dependent diabetes mellitus. Diabetes Res Clin Practice 1: 309
- Lucas MJ (2001) Diabetes complicating pregnancy. Obstet Gynecol Clin North Am 28: 513-36
- Miller E, Hare JW, Cloherty JP (1981) Elevated maternal hemoglobin A1C in early pregnancy and majorcongenital anomalies in infants of diabetic mothers. N Engl J Med 304: 1331-4
- Moore T. (2004) Diabetes in pregnancy. In: Creasy RK, Resnik R, Iams JD(editors). Maternal-fetal medicine: principles and practice. 5th ed. Philadelphia: Saunders, PP 1023-61
- Murry RK, Graner DK, Mayes PA, Rodwell VW (2003) Harper’s Illustrated Biochemistry. 26th ed. Mc Graw Hill: New York, PP 270-85
- Musen G, Lyoo IK, Sparks CR (2006) Effects of type 1 diabetes on gray matter density as measured by voxel-based morphometry. Diabetes 1(55): 326-33
- Pereira JA, Oliveira I, Sousa A, Valentao P, Andrade P, Ferreira I, et al (2007) Walnut (Juglans regia) leaves: phenolic compound, antibacterial activity and antioxidant potential of different cultivars. Food and Chemical Toxicology 45(11): 2287-95
- Persson B, Hanson U (1998) Neonatal morbidities in gestational diabetes mellitus. Diabetes Care 21:79-84
- Peter G Heppera,Leo R Leadera (1996) Fetal Habituation. Fetal and Maternal Medicine Review 8 (02):109-123
- Rahimi R, Nikfar Sh, Larijani B, Abdollahi M (2005) A review on the role of antioxidants in the management of diabetes and its complications. J Biomed Phar 59: 365 – 73
- Rakeshwar S. Guleria, Jing Pan, Donald DiPette and Ugra S. Singh (2006) Hyperglycemia Inhibits Retinoic Acid–Induced Activation of Rac1, Prevents Differentiation of Cortical Neurons, and Causes Oxidative Stress in a Rat Model of Diabetic Pregnancy. Diabetes 55(12): 3326-3334
- Rubelia Martı´nez-Tellezb, Ma. de Jesu´s Go´mez-Villalobosa, Gonzalo Flores (2005) Alteration in dendritic morphology of cortical neurons in rats with diabetes mellitus induced by streptozotocin. Brain Research 1048: 108 – 115
- Sermer M, Naylor CD, Farine D, Kenshole AB, Ritchie JW, Gare DK, et al (1998) The Toronto tri-hospitalgestational diabetes project. A preliminary review. Diabetes Care 2: 33-42
- Singh BS, Westfall TC, Devascar SU (1997) Maternal diabetes-induced hyperglycemia and acute intracerebral hyperinsulinism suppressfetal brain neuropeptide Y concentrations. Endocrinology 138(3): 963-9
- Thakran S, Siddiqui MR, Baquer NZ (2004) Trigonella foenum graecum seed powder protects against histopathological abnormalities in tissues of diabetic rats. Molecular and Cellular Biochemistry 266: 151–9
- Tripathi UN, Chandra D (2010) Anti-hyperglycemic and anti-oxidative effect of aqueous extract of Momordica charantia pulp and Trigonella foenum graecum seed in alloxan-induced diabetic rats. Indian J Biochem Biophys 47(4): 227-33
- Vermeulen MJ, Shapiro JL (2004) Preconception care of women with diabetes. Diabetes Care 27: S78- S91
- Wyngaarden JB, Smite LH (1982) Cecil Textbook of medicine.16th ed. W.B. Saunders co: Philadelphia PP: 1053-71
- Xue WL, Lei J, Li X, Zhang R (2011) Trigonella foenum graecum seed extract protects kidney function and morphology in diabetic rats via its antioxidant activity. Nutr Res 31(7): 555-62
- Xue WL, Li XS, Zhang J, Liu YH, Wang ZL, Zhang RJ (2007) Effect of Trigonella foenum-graecum (fenugreek) extract on blood glucose, blood lipid and hemorheological properties in streptozotocin-induced diabetic rats. Asia Pac J Clin Nutr 16: 422-6
- Zhen-Guo Li, Weixian Zhang, George Grunberger, Anders A.F Sima (2002) Hippocampal neuronal apoptosis in type 1 diabetes. Brain Research 946(2): 221–231
- Zia T, Hasnain SN, Hasan SK (2001) Evaluation of the oral hypoglycemic effect of Trigonella foenum-graecum L. (methi) in normal mice. Journal of Ethnopharmacology 75(2-3): 191-5








