Manuscript accepted on :
Published online on: 11-01-2016
Plagiarism Check: Yes
Ehsan Barati*1, Sara Soleimani Asl2, Seyed Ali Pourbakhsh3, Mahmoud Jamshidian3 and Siamak Shahidi4
1Student of Veterinary Microbiology, Science and Research Branch, Islamic Azad University, Tehran, Iran 2Department of Anatomy, School of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran 3Department of Microbiology ,Science and Research Branch, Islamic Azad University, Tehran, Iran 4Department of Physiology, School of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran
DOI : https://dx.doi.org/10.13005/bpj/845
Abstract
Alzheimer’s disease (AD) is a neurodegenerative disorder and most common form of dementia that leads to memory impairment. In the present study we have examined the investigating effect of Borago officinale on hipocampla IL-1 Beta protein in the Beta Amyloid peptid induced of inflammation in the rat. Wistar male rats received intrahippocampal (IHP) injection of the Aβ(25–35) and borage extract throughout gestation (100 mg/kg).inflamation was confitmed by pathologist.for mesearing the expression of the IL-1 Beta gene we used RT- PCR method and for extraction of the IL-1 Beta protein we used western bloting method.result showed that using Borago officinale lead to reduce IL-1 Beta gene and protein. This data suggests that borage could improve the reduction of inflammation factors such as IL-1 Beta and borage consumption may lead to an improvement of AD-induced cognitive dysfunction.
Keywords
IL1-Beta; Alzheimer; RT-PCR; Western Blot; Amyloid β-Peptide
Download this article as:| Copy the following to cite this article: Barati E, Asl S. S, Pourbakhsh S. A, Jamshidian M, Shahidi S. Investigating the Effect of Borago Officnale on Hipocampal IL-1 Beta Protein and Gene in the Amyloid Β-Peptide (25–35)-Induced of Inflammation in Rat. Biomed Pharmacol J 2015;8(2) |
| Copy the following to cite this URL: Barati E, Asl S. S, Pourbakhsh S. A, Jamshidian M, Shahidi S. Investigating the Effect of Borago Officnale on Hipocampal IL-1 Beta Protein and Gene in the Amyloid Β-Peptide (25–35)-Induced of Inflammation in Rat. Biomed Pharmacol J 2015;8(2). Available from: http://biomedpharmajournal.org/?p=3908 |
Introduction
In Alzheimer’s disease (AD) which is along with inflammation response of immune system in the brain, the increasing of complement proteins, cytokins, microglia cells and astrocyts is observed [1]. Cytokins are a big and heterogeneous group of healthy proteins that are produced by different cells and do the intermediation and setting of all aspects of natural and acquired immunes [2]. Some of these cytokins cause inflammation in the body that we can refer to IL-1 Beta. Perhaps IL-1 Beta can considered as the most important inflammatory cytokins. Monocyts and Macrophage are the most important IL-1 Beta producing cells [3].
The studies shows that in Alzheimer’s disease the Beta amyloid setting around the nerve cells lead to microglias activity , producing of free radicals the inflammatory process and hurts to healthy norons [4].
A lot of studies have been done on the effects of anti-inflammatory of Borage such as the investigations that Mattson et al [5] did and found that oil of Borage causes the reduction of inflammation because of the existence of GLA and increasing of the synthesis of prostaglandin E and cAMP.
Deshpande [6] studied the Herbal treatment of arthritis romatoid disease and emphasized that Borage reduces the rate of inflammatory cytokins because of the existence of GLA.
In this study it is tried to examine the effect of Borago Officinale in decreasing inflammation and decreasing of inflammatory factors such as IL-1 Beta by using peptid beta amyloid and creating Alzheimer’s model in rat.
Materials and Methods
The Aβ (25–35) was purchased from sigma-Aldrich company (St Louis, MO, USA). Borago officinalis leaves were obtained in dried condition from Research Institute for Islamic and Complementary Medicine (Tehran, Iran). Aβ25–35 was solubilized in sterile water at 1 μg/μL concentration and stored at −20°C.
Animals
28 male Wistar rats (Pasteur-Iran), weighing 250–300 g were included in this experimental study. All animals were group-housed and given ad libitum access to food and water. Housing conditions were maintained at a temperature of 2 1 ± 2 °C and the relative humidity of 5 0 ± 5 % on a 12 h light/12 h dark cycle [7].
The rats randomly were specified to the following groups: the control or intact group (= 7) that was left undisrupted; the sham-operated group; the Aβ25–35 model group which received single bilateral intrahippocampal (IHP) injections of Aβ25–35; the borage-treated group that received borage extract (orally, 100 mg/kg) following IHP injection of Aβ25–35 for 14 days [5].
Preparation of Borage Extract
Dried borage leaves were cleaned and ground into coarse powder by electrically driven device. The powdered material was soaked into aqueous ethanol (80%) for one week with occasional shaking .The extract was filtered through a Whatman filter paper and evaporated to dryness under reduced pressure at a maximum of 40°C using a rotary evaporator. Borago officinalis produced 10.9% dried extract. The extract was completely dissolved in purified water and kept at 4°C [8].
Intrahippocampal Injection of Aβ25–35
The animals were anesthetized with the ketamine (100 mg/kg) and xylazine (10 mg/kg) and were transferred to a stereotaxic apparatus (Stoelting, Wood Dale, IL, and USA). Injection was made using a 10 μL microsyringe (Hamilton-Reno, NV, USA). Relative to the bregma and with the stereotaxic arm at 0°, the coordinates for the toothed gyrus were posterior −3.6; lateral ±2.3; and dorsal 3 mm [9].
Aβ solution (6 μL) was bilaterally injected into the area over 1 μL/2 min. the cannula was left in place for 2 min after each injection to allow for spread. Sham operated rats received vehicle solution [10]. The skin was then stitched and the animals were left to recover in a warm box before returning to their home cages. The injection place was checked by injection trypan blue instead of peptide in preliminary experiments (Figure 1).
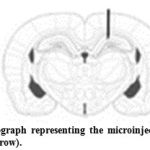 |
Figure 1: Schematic photograph representing the microinjection site of Amyloid β into the hippocampus (black arrow). |
Histological Verification
For verification of injection place a light microscope (Olympus, Japan), the trypan blue injected rats were perfused with 4% paraformaldehyde in 0.1 M phosphate buffer (p H = 7. 3) and the hippocampi were serially sectioned into 10 μm coronal parts by a microtome (Leica Instruments, Germany). After deparaffinization and rehydration, sections were stained in 0.1% cresyl violet for 3 minutes. Finally, the parts were photographed with a digital camera (Olympus, DP 11, Japan) attached to a microscope (Olympus Provis, Ax70, Japan) and the stained pieces were qualitatively analyzed for the injection site [10].
RT-PCR
RNA Extraction
The rat hipocampal tissues were kept at -70degree centigrade. Total RNA from 30 mg of rat tissue using Trizol reagent (Invitrogen) was extracted.
The process was as follows: After adding 1 mL of Trizol reagent in the tissues of the rat, the tissue was crushed using homogenizer and then centrifuged at 14,000 RPM and 200 μL of chloroform was added. After mixing the supernatant fluid with the same amount of isopropanol, the reaction was done at room temperature.
After a reaction, total RNA at 14,000 RPM was centrifuged; precipitate was obtained and washed by 75% ethanol. The total RNA was examined by Bioanalyzer 2100 (Agilent Technologies Inc.) and stored at -70 degree centigrade.
cDNA synthesis
One μg of the RNA was synthesized using AB High Capacity RNA-TO-cDNA Kit (Quiagen, Hilden, Germany).
At first, 1 μg of RNA volume was mixed with dextrose water to total volume 9 mL, it was then spinned down in ice for 1 minute at the temperature of 37degree centigrade for 60 minutes. Finally, it was heated at 95degree centigrade for 5 minutes. Synthesized cDNA was kept at -20 degree centigrade.
RT-PCR Analysis
RT-PCR was performed in triplicate in 384-well plates. The resulting first-stranded cDNA was normalized by the glyceraldehyde 3-phosphate dehydrogenase gene. The normalized cDNA was used for the PCR procedure as a template.
The PCR reaction was conducted in a final volume of 20 μL containing cDNA 0.5 μL, 0.8μL of primer (10 pM/μL) and 2× SYBR Green PCR Master Mix (Applied Biosystems), which included the HotStarTaqt DNA-polymerase in an optimal buffer 0.4 μL (5 U/μL), the dNTP mix (with dUTP additive) 1 μL (each 2.5 mM).
Thermal cycling conditions 50degree centigrade for 2 minutes and 95degree centigrade for10 minutes followed by 40 cycles of 95degree centigrade for 30 seconds and 60degree centigrade for 30 seconds and 72degree centigrade for 30 seconds. A melting curve analysis of products was performed routinely in order to exclude the presence of unspecific products, after finishing amplification by high resolution data collection during incremental temperature increase from 60degree centigrade to 95degree centigrade with a ramp rate of 0.21degree centigrade/sec. RT-PCR cycle numbers were converted to gene amounts (ng) on the basis of the equation. The RT- PCR analysis was done on an Applied Biosystems Prism 7900 Sequence Detection System (PE Applied Biosystems). The amount of each gene expression was revised by relative quantification assay.
The primers used for RT- PCR ampilifications are in the table below (Cinnagen, Tehran, Iran).
| Length of Sequence | Goal Gene | Primer | Annealing Temperature |
| 85 | IL1-Beta | Forward 5`- GGA TGA TGA CGA CCT GCT AGT GT-3`
Reverse 5`-TGG AGA GCT TTC AGC TCA CAT G -3` |
61
63 |
| 260 |
βActin |
Forward 5`- GTG GGC CGC CCT AGG CAC CAG-3`
Reverse 5`- GGC CTT AGG GTT CAG AGG GG-3` |
58
62 |
Western Blots
Protein Extraction
Protenins were extracted with 1000 μL of lysis buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% triton, 1 mM EDTA, 1/20 (v/v) anti-protease cocktail (Sigma-Aldrich, France) per 6 wells. The volume of lysate obtained was mixed with 4 volumes of methanol, 1 volume of chloroform and 2 volumes of water. After vortexing, thsamples were centrifuged for 5 min at 20 000 g. The upper phase was taken and mixed with 3 volumes of methanol and centrifuged as before. The pellet was resuspended in Tris 50 mM, Na Cl 145 mM, SDS 0.5%
pH 7.5.
Western blot Analysis
SDS-PAGE was performed according to the LaemmLi protocol (1970), under reductive conditions with 12.5% running gels and 4% stacking gels. Gels were run for 2 hours at 4°C and 15 V, and then were stored in bottles onto a nitrocellulose membrane (Millipore, France) using a liquid transfer system (Biorad). Membranes were soaked for 30 min in TBS buffer (138 mM NaCl, 15 mM Tris-base) containing 0.05% (v/v) Tween 20, 0.05% Triton, 5% BSA.
Total IL1-Beta protein was discovered with anti IL1-Beta antibody (Santa Cruz, Spain) at a 1/2000 Dilution and β-actin (Sigma, St. Louis, MO, USA). The membranes were incubated in TBS buffer (138 mM NaCl, 15 mM Tris-base) containing 0.05% (v/v) Tween 20, 0.05% Triton, 5% BSA with the primary antibody for 2 hours at room temperature. Membranes were washed 3 times for 10 min in TBS buffer (138 mM NaCl, 15 mM Tris-base) containing 0.05% (v/v) Tween 20, 0.05% Triton. This was followed by incubation with alkaline phosphatase-conjugated polyclonal anti-rabbit immunoglobulin (1/1000) in TBS buffer (138 mM NaCl, 15 mM Tris-base) containing 0.05% (v/v) Tween 20, 0.05% Triton, for one hour at room temperature. After four 5 min washes with TBS buffer, development was completed with an enzymatic assay (ECL, Amersham Biosciences)and visualized with a Kodak 2000R Image.
Statiscal Analyses
Data were presented as mean ± S.E.M and analyzed by SPSS version 21 software. The data of the IL-1 Beta protein and gene were analyzed using two-way analysis of variance (ANOVA), Tukey multiple comparisons tests were used to analyze the significance of the differences between the groups, when appropriate value of p<0.05 was considered significant [8].
Briefly, the Homogenous test was used to prove the variance homogeneity among the experiment groups.
Results
Histological analysis showed that injection of Aβ was in their desired location, according to the atlas of Paxinos and Watson (Figure 1) [9].
The study of the expression of IL-1 Beta gene
The obtained results from RT- PCR show that IL1-Beta gene has a significant reduction among the intact groups in comparison with Beta amyloid and Borage(p<0.05 for both groups of Beta amiloid), that show the increase for the expression of this gene among the Beta amyloid and Borage groups.
Priscriping the Borage caused a significant reduction in IL-1Beta gene after creating Alzheimer model.
The result obtained from the statistic analyzing IL-1Beta in attendance groups by ANOVA method showed that there is a significant difference among the experimenting groups.
The Tukey test was used because of the significant difference. The results of this test showed that a significant difference was not observed under the conditions of experiment among the Sham and Intact groups from the view point of IL-1Beta. Also a significant difference was observed between the Sham and Intact group in comparison with Beta amyloid and Borage groups.
In this test, a significant difference was observed between the Beta amyloid and Borage groups from the view point of IL-1Beta.
Briefly the Homogenous test was used to prove the variance homogeneity among the attendance groups that the result of this test was not significant among the attendance groups under the conditions of experiment, that it showed the variance homogeneity among the test groups.
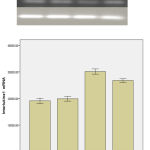 |
Figure 1 |
The study of the rate of the expression of protein in IL1-Beta
Densitometry, the nitro cellulose membrane of the protein in Borage and Beta amyloid showed that the rate of this protein in Borage and Beta amyloid groups had a significant increase in comparison with intact and Sham groups. The prescription of the Borage after creating Alzheimer model caused a significant reduction of the rate of the expression of the IL-1Beta protein in comparison with the Beta amyloid group.
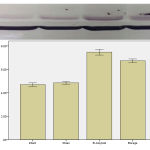 |
Figure 3 |
Discussion and Conclusion
The results obtained from the present study showed that the prescription of Beta amyloid leads to the increase of the expression of gene and protein IL1-Beta in hippocampus area. Also the prescription of Borage juice after creating Alzheimer model reduced the injected inflammation caused by Beta amyloid.
In present study the mutual injection in hippocampus Beta amyloid leads to creating inflammation and increasing inflammatory proteins. In agreement with our study Yamaguchi and et al. reported that prescription of Beta amyloid causes Alzheimer [11].
The various experimental studies reported the role of rich feeding from anti-inflammations and anti-oxidants in recovery of neuro toxicity and recovery of the act of the brain [12].
Great numbers of the medicinal plants were used in neurotic disease as anti-inflammation. Among these plants, the Borago Officinalis has a great importance because of having much Gama Linolenic Acid (GLA) [13].
Peng et al [14] in a research that they did about Borago Officinale, got this result that Borage reduces the danger of catching diseases such as cancer, diabet and Alzheimer.
In a research that Kapoor and et al did about the effect of GLA a natural anti-inflammation, got this result that GLA prevents producing of the IL-1 Beta and its releasing by monocyte and in this way causes the reduction of inflammation. Also GLA causes secretion of prostaglandin E and this factor finally causes the reduction of producing TNF-α and in this way the inflammation finally reduces [15].
Harbige et al [16] in a research that they did about Borago Officinale declared that this plant has more than 20% GLA and traditionally it is used in the treatment of Romatoid and has positive and useful effect on the brain.
Engler and Engler [17] in a research that they did about the anti-inflammation effect of the oil of Borago Officinale got this result that it causes the reduction of inflammation because of the existence of GLA and increasing of the synthesis of prostaglandin E and CAMP.
In a research that Deshpande [6] did about the Herbal treatment of arthritis romatoid disease, emphasized that Borago Officinale reduces the rate of inflammatory cytokins because of the existence of GLA.
The result of this study showed that using the Borage juice after the injection of Beta amyloid lead to the reduction of inflammation. In the present study after analyzing the results of the experiment by SPSS software version 21, it was confirmed that the surgical operation has had no effect on the results of the experiment. By the injection of Beta amyloid in hippocampus, the rate of the gene expression and protein IL1-Beta in the Borage and Beta amyloid group get a significant increase in comparison with the Sham and Intact groups which this is a confirmation on the created inflammation after the injection of Beta amyloid. After the consumption of the alcolic juice of Borago Officinale the rate of the gene expression and hippocampic IL1-Beta protein get a significant reduction in comparison with Beta amyloid group which it is the confirmation in the hypothesis of the present study which declars that the consumption of Borago Officinale casues the reduction of the created inflammatory factors in the Alzheimer model which is created by peptid Beta amyloid in hippocampus.
Generally it seems that the Borago Officinale reduces the effect of inflammatory proteins and in this way it reduces the effects of inflammatory Beta amyloid.
Briefly, the results of the present study declare that the prescription of the Borago Officinale reduces the injected inflammation by Beta amyloid and therefore it may be useful in the treatment of some disease as Alzheimer.
References
- Walsh, D.M., Teplow, D.B. Alzheimer’s disease and the amyloid b-protein. Prog Mol Biol Transl Sci., 2012; 107: 101-124.
- Choi, S.S., Lee, S.R., Kim, S.U., Lee, H.J. Alzheimer’s disease and stem cell therapy. Exp Neurobiol., 2014; 23(1): 45-52. doi.org/10.5607/en.2014.23.1.45
- Rinne, J.O., Kaasinen, V., Järvenpää, T., Någren, K., Roivainen, A., Yu, M., … Kurki, T. Brain acetylcholinesterase activity in mild cognitive impairment and early Alzheimer’s disease. J Neurol Neurosurg Psychiatry., 2003; 74(1): 113-5. doi.org/10.1136/jnnp.74.1.113
- Sarter, M., Bruno, J.P. Developmental origins of the age-related decline in cortical cholinergic function and associated cognitive abilities. Neurobiol Aging., 2004; 25(9):1127–39. doi.org/10.1016/j.neurobiolaging.2003.11.011
- Mattson, M.P., Maudsley, S., Martin, B. A neural signaling triumvirate that influences ageing and age-related disease: insulin/IGF-1, BDNF and serotonin. Ageing Res Rev., 2004; 3(4): 445–64. doi.org/10.1016/j.arr.2004.08.001
- Deshpande, A. Different Conformations of Amyloid beta Induce Neurotoxicity by Distinct Mechanisms in Human Cortical Neurons. J Neurosci., 2006; 26(22): 6011–8. doi.org/10.1523/jneurosci.1189-06.2006
- Shankar, G.M., Li, S., Mehta, T.H., Garcia-Munoz, A., Shepardson, N.E., Smith, I., et al. Amyloid-β protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med., 2008; 14(8): 837-842. doi.org/10.1038/nm1782
- LaFerla, F.M., Tinkle, B.T., Bieberich, C.J., Haudenschild, C.C., Jay, G. The Alzheimer’s Aβ peptide induces neurodegeneration and apoptotic cell death in transgenic mice. Nat Genet., 1995; 9(1): 21-30. doi.org/10.1038/ng0195-21
- Conforti, F., Sosa, S., Marrelli, M., Menichini, F., Statti, G.A., Uzunov, D., et al. In vivo anti-inflammatory and in vitro antioxidant activities of Mediterranean dietary plants. J Ethnopharmacol., 2008; 116(1): 144–51. doi.org/10.1016/j.jep.2007.11.015
- Klementiev, B., Novikova, T., Novitskaya, V., Walmod, P.S., Dmytriyeva, O., Pakkenberg, B., et al. Corrigendum to “A neural cell adhesion molecule-derived peptide reduces neuropathological signs and cognitive impairment induced by Aβ25–35. Neurosci., 2007; 145(1): 209–224. doi.org/10.1016/j.neuroscience.2014.02.010
- Zussy, C., Brureau, A., Delair, B., Marchal, S., Keller, E., Ixart, G., et al. Time-Course and Regional Analyses of the Physiopathological Changes Induced after Cerebral Injection of an Amyloid β Fragment in Rats. Am J Pathol., 2011; 179(1): 315–34. doi.org/10.1016/j.ajpath.2011.03.021
- Cappai, R., Barnham, K.J. Delineating the Mechanism of Alzheimer’s Disease Aβ Peptide Neurotoxicity. Neurochem Res., 2007; 33(3): 526–32. doi.org/10.1007/s11064-007-9469-8
- Yamaguchi, Y., Kawashima, S. Effects of amyloid-β-(25–35) on passive avoidance, radial-arm maze learning and choline acetyltransferase activity in the rat. Eur J Pharmacol., 2001; 412(3): 265–72. doi.org/10.1016/s0014-2999(01)00730-0
- Peng, Q., Buz’Zard, A., Lau, B.H., Pycnogenol® protects neurons from amyloid-β peptide-induced apoptosis. Mol Brain Res., 2002; 104(1):55–65. doi.org/10.1016/s0169-328x(02)00263-2
- Kappor, D., Foster S, Tyler V. E. Tyler’s Honest Herbal: A Sensible Guide to the Use of Herbs and Related Remedies. New York: Routledge. 1999.
- Harbige, L.S., Layward, L., Morris-Downes, M.M., Dumonde, D.C., Amor, S. The protective effects of omega-6 fatty acids in experimental autoimmune encephalomyelitis (EAE) in relation to transforming growth factor-beta 1 (TGF-beta1) up-regulation and increased prostaglandin E2 (PGE2) production. Clin Exp Immunol 2000; 122(3): 445–52. doi.org/10.1046/j.1365-2249.2000.01399.x
- Engler, M.M., Engler, M.B. Dietary borage oil alters plasma, hepatic and vascular tissue fatty acid composition in spontaneously hypertensive rats. Prostaglandins Leukot Essent Fatty Acids., 1998; 59(1):11–5. doi.org/10.1016/s0952-3278(98)90046-1







