Anoosheh Leila1*, Kordi Mohammad Reza2, Gaeini Abbasali2, Mahdian Reza3, Mirakhori Zahra1
1Exercise Physiology Ph.D., Faculty of Physical Education and Sports Sciences, University of Tehran, Tehran, Iran 2Faculty of Physical Education and Sports Sciences, University of Tehran, Tehran, Iran 3Department, Biotechnology Research Center, Pasteur Institute of Iran, Tehran, Iran Corresponding Author Email : l.anoosheh@ut.ac.ir
DOI : https://dx.doi.org/10.13005/bpj/827
Abstract
The purpose of the present research was to determine the effects of aerobic exercise on NF-kB, Lin28B, let-7a microRNA and IL-6 of tumor tissue in mice with breast cancer. 20 female BALB/c mice were injected with MC4-L2 estrogen-receptor-positive breast-cancer cells, then divided into two groups of 10 mice each: tumor-exercise (TE) and tumor-control (TC). The TE group performed aerobic exercise five days per week for six weeks. Widths and lengths of the tumors were measured weekly using digital calipers. The mice were sacrificed 48 hours after the last exercise session. Expression of NF-kB, Lin28B and miRNA let-7a genes were quantified with Real time-PCR and IL-6 was measured by the ELISA test. Tumor volume, expression of NF-kB and Lin28B genes and IL-6 levels diminished significantly in the TE group compared with the TC group, but let-7a microRNA expression showed a significant increase in the TE group compared with the TC group (P<0.05). The results demonstrate that aerobic exercise can have an effective role in the diminution of IL-6, Lin28B and NF-kB, and the increase of microRNA let-7a and it seems that regular aerobic training may at least have a therapeutic role in estrogen-receptor-dependent breast cancers. However, more studies are needed.
Keywords
Estrogen-receptor-dependent breast cancer; Aerobic training; NF-kB; Lin28B; microRNA let-7a
Download this article as:| Copy the following to cite this article: Leila A, Reza K. M, Abbasali G, Reza M, Zahra M. Effects of Exercise Training on Development of Breast Cancer in Mice. Biomed Pharmacol J 2015;8(2) |
| Copy the following to cite this URL: Leila A, Reza K. M, Abbasali G, Reza M, Zahra M. Exercise Training and Development of Breast Cancer. Biomed Pharmacol J 2015;8(2). Available from: http://biomedpharmajournal.org/?p=1826 |
Introduction
Inflammatory response has a pivotal role in the tumorigenesis, progress and metastasis of most cancers, particularly breast cancer (1). Breast cancer is the most health-threatening factor for females, as it is the most common cancer in this gender group (2). Breast cancer is of several major molecular subtypes, which are classified as either estrogen-dependent or estrogen-independent. Most breast cancers are epithelial tumors arising from the cell linings of breast ducts or lobules of breast, and are called estrogen α-receptors positive [ER α positive] (3).
It has been demonstrated that an epigenetic switch, from normal breast cell to cancerous, is activated by an inflammatory signal, and epigenetic inheritance is mediated by a positive feedback loop involving the nuclear factor kappa-light-chain-enhancer of activated B class (NF-kB), Lin28 homolog B (Lin28B) protein, Lethal-7a (let-7a) microRNA and Interleukin-6 (IL-6). This regulatory circuit links inflammation to cellular transformation (4).
NF-kB is a transcription factor regulating the expression of anti-apoptosis genes that also activates pre-inflammatory chemokines and cytokines. In fact, NF-kB is a key mediator of cancerogenesis induced by inflammation. NF-kB prevents expression of let-7. Considering this view, preventing the expression of NF-kB leads to incremental expression of let-7 (5). Let-7 is a family of miRNA consisting of 12 members in the human genome that has a close relationship with cancers of all types. Let-7a is a member of let-7, which acts as a suppressor (1). In confirming this, studies have demonstrated that diminution of the let-7a gene is accompanied by increased tumorigenesis. Moreover, increased gene expression causes decreased implanted tumor cells and the tumor itself (6, 7).
Because NF-kB acts as an activator protein, direct inhibition of let-7a expression appears impossible. Instead, it is proposed that NF-kB may activate an inhibitor of let-7a, such as Lin28B, that strongly prevents expression of let-7 in both the transcription and post-transcription stages. Lin28 is a protein expressed in two forms – Lin28A and Lin28B – which can be linked to pre let-7 and prevent the production of let-7 (8).
Thus, NF-kB, by using Lin28B as a mediator, immediately transmits an inflammatory signal to a mechanism that eventually inhibits let-7a expression. In contrast, IL-6, a main mediator of inflammatory response, is directly suppressed by let-7a. It has been shown that IL-6 is increased in epithelial cancers such as breast carcinoma(9). IL-6 is a cytokine that has a pre-inflammatory function in the tumor micro-environment and affects – and metastasis (10) as well as angiogenesis (the creation of new blood vessels that increase the blood flow, and therefore growth, of the tumor (11)).
Although it has been demonstrated that inflammation diminishes following aerobic exercise, the existing results, particularly in the domain of cancer, are not exclusive. There is a question whether aerobic exercise can decrease the activity of the positive feedback loop between NF-kB, Lin28B, let-7a and IL-6, and eventually reduce inflammation and tumor growth in mice with breast cancer. Therefore, this research aimed to investigate the effects of a course of moderate-intensity aerobic exercise on NF-kB, Lin28B, let-7a and IL-6 in the tumor tissue of mice with breast cancer.
Materials and Methods
Subjects
Twenty BALB/c (four to five weeks old and weighing 15-17g) female mice were purchased from Pasteur Institute and kept five to a cage in 22-23 ºC and 45% humidity. Twelve hours of light and 12 hours of darkness (light from 6 am, darkness from 6 pm) were applied for physiologic adaptation of the mice. The animals’ feed was the usual mice feed, which they could access ad lib up to the end of the protocol.
Exercise Protocol
All of the mice were familiarized with the living conditions of the animal house and the manner of running on the treadmill for one week. They were then inoculated with the cancer cells, and after 10 days, were divided into two 10-mouse groups: the tumor-control (TC) group, which performed no exercise, and the tumor-exercise (TE) group, which performed moderate-intensity aerobic exercise five days a week for six weeks, as shown in Table 1. The protocol consisted of aerobic exercise; specifically, running on a treadmill (3). This exercise was used because the investigator could easily regulate its intensity and duration. Because doctors and specialists have recommended that exercise intensity should be at an efficacious and safe level, and that an exercise program that would normally be considered mild or moderate may be high for cancer patients(3), in this study, moderate-intensity exercise (55-70 percent VO2max) was applied. The different intensities of exercise were based on the research conducted by Agha-Alinezhad in 2008 (12).
Table 1: Aerobic exercise protocol on treadmill
| Exercise period | Velocity (m/min) | Time (min) | Repetition (Day/Week) |
| Familiarization step | 6-10 | 20 | 5 |
| First 2 weeks | 14 | 25 | 5 |
| Second 2 weeks | 16 | 30 | 5 |
| Third 2 weeks | 18 | 30 | 5 |
Cell Culture
As the majority of breast cancers are known as ER-α positive (3), Carcinoma cells of estrogen-positive breast cancer (MC4-L2) cell line, a murine ER-α positive cell line, was used in our study. MC4-L2 cell line were obtained from Lanari, an investigator from Buenos Aires University in Argentina (13). The MC4-L2 cells were cultured in a T75 flask in a DMEM/F-12 environment added to 15 mM of HEPES buffer, glutamine, 100 μg/ml of penicillin, 100 μg/ml of streptomycin, and 10% FBS. After 90% of the flask was filled with cells, the supernatant was withdrawn and, after washing with PBS, it was separated from the cell plate using 0.025 trypsin enzyme; then, following neutralization of the enzyme in an environment containing 10% FBS, the entire contents of the flask were poured into a Falcon tube and centrifuged at 1200 rpm for 3-5 minutes; in the subsequent stage, the supernatant was withdrawn and the cell plate was dissolved in an environment containing 10% FBS. Then, trepan blue was used to determine the cells’ viability and hematocytometer was used to count them (3).
Tumor formation
Female BALB/c mice were anesthetized using a suitable dose of ketamine and xyloxine, and one million cells were injected subcutaneously into each mouse’s right upper thigh. Approximately 10-14 days after the injection of cancer cells, the tumor was palpable in the injected area. After the tumor emerged, its length (L) and width (W) were measured every week. The formula of Jones et al., V=π/2(L2xW), was used to calculate the tumor volume (14).
IL-6 Measurement
Forty-eight hours after the last exercise session, the mice of both groups were sacrificed. The tumor tissue was removed immediately and its central necrotic part was discarded. The superficial part of the tumor was frozen in liquid nitrogen at -70°C. Tumor tissue was homogenized with lyses solution, total protein was extracted using the Bradford method, and the supernatant was used for ELISA. IL-6 was measured and quantified using ELISA based on the kit instructions. The Ab100713 ELISA kit by Abcam was used to measure IL-6.
RNA isolation and cDNA Synthesis
RNA isolation stages were performed based on the Trizol protocol of Life Technology Co. of the United States. To isolate let-7 microRNA, after adding isopropanol, the supernatant was kept at 20 ºC for 24 hours and the subsequent stages of isolation were performed (15). The Qiagen kit was used for NF-kB and Lin28B cDNA Synthesis and the Stratagen kit, made by Agilent Technology Co. of the United States, was used for let-7a cDNA synthesis according to the company’s protocol.
Real-Time PCR
The Real-Time PCR program was used on Model 5 plex HRM Corbett apparatus manufactured in Australia for Lin28B and NF-kB genes at 40 cycles per minute for the following configurations: 95 ºC for 10 minutes, 95 ºC for 15 seconds, 60 ºC for one minute, and, for let-7a at: 95 ºC for 10 minutes at 45 cycles per minute, 95 ºC for 10 seconds, 60 ºC for 15 minutes and 72 ºC for 20 seconds. The expression of GAPDH was also determined as an internal control of Lin28B and NF-kB, and the expression of U6 was used as the control the gene let-7a (3).Data analysis
In the SPSS19 software, repeated measures of ANOVA and the student’s t test were used to assess tumor volume and IL-6, respectively. Statistical analysis of NF-kB, Lin28B and let-7a was performed by REST[1].
[1] Relative expression software tool.Results
The results of repeated measures ANOVA demonstrated a significant difference between the tumor growth of the two groups (F=9.7, P=0.001). The growth process in the protocol that included six weeks; is shown in the figure1. The final tumor growth and the growth process in the tumor-control group were higher than those in the tumor-exercise group. The original tumor volume in both the tumor-exercise (TE) and tumor-control (TC) groups were relatively equal, but the final growth rate of the tumor eventually diminished in the TE group.
Table 2: Utilized primers
| Genes | NCBI | Forward Sequence | Reverse Sequence |
| let-7a | NR_029725 | —- | UGAGGUAGUAGGUUGUAUAGUU |
| U6 | NR_003027 | G TGCAGGGTCCGAGGT | GCGCGTCGTGAAGCGTTC |
| NF-kB | NM_008689 | ATCACTTCAATGGCCTCTGTGTAG | GAAATTCCTGATCCAGACAAAAAC |
| Lin-28 | NM_145833.1 | CTTCCATGTGCAGCTTGCTCT | GTTCGGCTTCCTGTCTATGACC |
| GAPDH | NM_008084 | ACCCTGTTGCTGTAGCCGTATTC | TCAACAGCAACTCCCACTCTTCC |
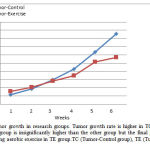 |
Figure 1: Rate of tumor growth in research groups. Tumor growth rate is higher in TC group, initial tumor volume in TE group is insignificantly higher than the other group but the final growth rate has been reduced following aerobic exercise in TE group.TC (Tumor-Control group), TE (Tumor-Exercise group). |
The data gained using quantitative Real-Time PCR was analyzed by REST. The results showed a significant reduction of Nf-kB following aerobic exercise training. Expression of Lin28B also significantly decreased. The data demonstrated a significant increase of let-7a expression in the TE group compared with the TC group. The ratio of the expression of let-7a in the TE group to that of the TC group was 21.37 (Table 3).
Table 3: Results of expression ratio of variables in tumor-exercise compared to tumor-control groups
| Expression ratio of tumor-exercise groups to tumor-control groups
|
P | Results | |
| Nf-kB | 0.128 | 0.004* | DOWN |
| Lin28B | 0.305 | 0.001* | DOWN |
| Let-7a | 21.366 | 0.000* | UP |
*significant difference between groups (A P value <0.05 was considered significant)
Also, the student’s t-test showed a significant difference in the IL-6 of the two groups (t=3.11, P: 0.007). The amount of IL-6 in the TC group was 1.6 times that in the TE group (Figure 2).
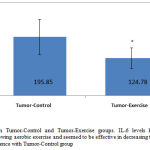 |
Figure 2: IL-6 levels in Tumor-Control and Tumor-Exercise groups. IL-6 levels have been diminished significantly following aerobic exercise and seemed to be effective in decreasing tumor volume. *significant difference with Tumor-Control group |
Discussion
In the present investigation, diminution of tumor volume in the TE group compared to the TC group was observed following aerobic exercise training. The mechanisms of the effects of exercise on tumor volume are complex. Inflammatory status is one of the mechanisms involved in tumor growth; it has been shown that aerobic exercise training can reduce this status. In this study, the levels of IL-6, which act as an inflammatory cytokine in the tumor tissue, were assessed. The results showed that, following aerobic exercise training, the levels of this cytokine diminished significantly and seemed to be effective in decreasing tumor volume. IL-6 cytokine activates a wide spectrum of messaging routes that lead to angiogenesis. Investigations show that these cytokines induce activation of messaging routes such as Nf-kB in the tumor tissue, which ultimately lead to stimulation of blood vessels’ endothelial cells and cause angiogenesis and increased tumor growth of (4).
Other studies have also reported diminution of tumor volume following regular sport exercise (3, 16, 17). Murphy et al. (2011) reported reduction of tumor volume in mice with breast cancer following 20 weeks of aerobic exercise, attributing the reduction to lowered levels of pre-inflammatory cytokines such as IL-6; they reported the presence of a direct relationship between pre-inflammatory cytokines and tumor volume (17). Zielinski et al. (2004), too, showed that intensive activity affects tumor growth by affecting the micro-environment of the tumor, and leads to delayed tumor growth (16). Verma et al. (2009) also observed a reduction in tumor growth and related it to the reduction of angiogenesis, reduced expression of VEGF, erythrocyte count and lactates in the micro-environment of the tumor and increased levels of oxygen and nitric oxide (18).
The present research also showed significantly reduced expression of Nf-kB in the tumor-exercise group compared with the tumor-control group following six weeks of aerobic exercise. In the domain of the effects of exercise training on the expression of Nf-kB in breast cancer, no research has been conducted thus far. The effect of one bout of exercise on the expression of Nf-kB in healthy individuals has been surveyed in only a few studies (19, 20). In the investigation conducted by Cuevas et al. (2005), expression of Nf-kB significantly increased following a bout of super-maximal aerobic exercise in professional bike riders. Vider et al. (2001) observed a significant increase of Nf-kB in blood macrophages following one bout of exercise with 80% VO2max (21). Discrepancies between the results of these studies and those of the present research can be justified as follows: first, in both of the mentioned studies, the effect of only one bout of exercise had been investigated, while in the present investigation, the effects of six weeks of aerobic exercise on the expression of Nf-kB was measured. Second, the types of exercise applied in the studies were different: maximal and super-maximal sport activities in the earlier studies, and moderate intensity aerobic exercise in the present investigation.
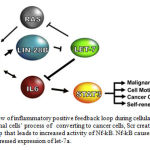 |
Figure 3: Schematic overview of inflammatory positive feedback loop during cellular transformation (Iliopoulos et al. 2009). In normal cells’ process of converting to cancer cells, Scr created an inflammation starting signaling for the loop that leads to increased activity of Nf-kB. Nf-kB causes increases in Lin28B, which in turn leads to suppressed expression of let-7a. |
In the present investigation, a significant reduction in the expression of Lin28B and a significant increase in the expression of let-7a were observed in the tumor-exercise group compared with the tumor-control group. Frequency studies have demonstrated that the expression of let-7a is reduced in many cancers. In an animal-model study (mice with breast cancer), inoculation of let-7a induces decremental regulation of Ras and HMGA2 oncogens (22). It has been reported that let-7a may target estrogen receptors and, potentially, prevent signaling of estrogen in estrogen-receptor-positive breast cancers. Also, it has been demonstrated that let-7a can prevent tumor-growth angiogenesis and metastasis in breast cancer by suppressing the expression of Angiogenin, Fibroblast Growth Factor (FGF), Matrix Metalloproteinase (MMP) and IL-6 (23). It appears that let-7a is a molecular marker in special cancers and has the capacity to be applied as a therapeutic method in the treatment of some cancers (24).
To date no research has examined the effects of exercise on let-7a and Lin28B in breast cancer, but the results of the investigation conducted by Simon et al. (2006) indicate that one of the reasons for diminished IL-6 following exercise training in healthy people is an increase in let-7 (24). Micro-RNA let-7 can, directly and indirectly, prevent gene expression of IL-6 (4). The present research also shows decreases in IL-6 and increases in let-7; one can attribute part of the reduction in IL-6 to increased let-7a following aerobic exercise.
However, as observed in Figure-1, IL-6 is an element of positive feedback loop in the emergence of breast cancer, and let-7a, Lin28B and Nf-kB are other elements of it (4). It appears that diminution of Nf-kB following a course of aerobic exercise, observed in the present study, may have a role in the diminution of Lin28B and, consequently, escalation of expression of let-7a.
In the research conducted by Iliopoulos et al. (2009), in normal cells’ process of converting to cancer cells, Scr created an inflammation starting signaling for the loop that leads to increased activity of Nf-kB. Nf-kB causes increases in Lin28B, which in turn leads to suppressed expression of let-7a. Thus, Nf-kB can increase IL-6 by suppressing the expression of let-7a both directly and indirectly. Increase of IL-6 induces more escalation of Nf-kB and ultimately leads to the activation of STAT3. STAT3 is a transcription factor that activates growth factors such as vascular endothelial growth factor (VEGF), and has a role in breast-cancer tumorigenesis (25, 26).
Conclusion
The results of the present investigation demonstrate that regular aerobic exercise training can have an effective role in reducing Lin28B and NF-kB and increasing microRNA let-7a. As it has been demonstrated that perturbation of any component of the regulatory circuit (inhibition of NF-kB, Lin28B or IL-6 or overexpression of let-7a) could induce significant reduction of tumor growth and mortality in breast cancer, it could be claimed that regular aerobic training may at least have a therapeutic role in estrogen-receptor-dependent breast cancers. In general, the findings of the present study propose that regular aerobic exercise can be applied as an adjuvant therapeutic method along with other methods of therapy in breast cancer. However, more studies are needed.
Acknowledgement
The present investigation was conducted with the financial sponsorship of the Physical Education Faculty of the University of Tehran and the contribution of Tarbiat Modares University. We take the opportunity to acknowledge all those who contributed to this research.
References
- Taniguchi K, Karin M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Seminars in Immunology. 2014;26: 54–74.
- Siegel R, Naishadham D, Jemal A. Cancer statistics. CA: A Cancer Journal For Clinicians. 2013;63:11-30.
- Amani-Shalamzari S, Aghaalinejad H, Alizadeh S, Kazmi A, Saei MA, Minayi N, Shokrolahi F. The effect of endurance training on the level of tissue IL-6 and VEGF in mice with breast cancer. Journal of Shahrekord Uuniversity of Medical Sciences. 2014;16: 10-21.
- Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-κB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139: 693-706.
- Woods JA, Vieira VJ, Keylock KT. Exercise, inflammation, and innate immunity. Immunology and allergy clinics of North America. 2009;29: 381-93.
- Ross SA, Davis CD. MicroRNA, nutrition, and cancer prevention. Advances in Nutrition: An International Review Journal. 2011;2: 472-85.
- Boyerinas B, Park S-M, Hau A, Murmann AE, Peter ME. The role of let-7 in cell differentiation and cancer. Endocrine-Related Cancer. 2010;17: 19-36.
- Viswanathan SR, Daley GQ, Gregory RI. Selective blockade of microRNA processing by Lin28. Science. 2008; 320: 97-100.
- Faragollahi F. Strategies used by patients receiving chemotherapy to relieve fatigue. Iran Journal of Nursing. 2004;17: 58-64
- Hong DS, Angelo LS, Kurzrock R. Interleukin‐6 and its receptor in cancer. Cancer. 2007;110: 1911-28.
- Thompson HJ, Jiang W, Zhu Z. Candidate mechanisms accounting for effects of physical activity on breast carcinogenesis. IUBMB Life. 2009;61: 895-901.
- Agha-Alinejad H, Tofighi A, Hassan ZM, Mahdavi M, Shahrokhi S. The effect of continuous endurance training on serum HSP70 level and life span of mouse bearing breast cancer tumors. Olympic. 2008;42: 75-86.
- Lanari C, Lüthy I, Lamb CA, Fabris V, Pagano E, Helguero LA, Sanjuan N, Merani S, Molinolo AA. Five novel hormone-responsive cell lines derived from murine mammary ductal carcinomas: in vivo and in vitro effects of estrogens and progestins. Cancer Research. 2001;61: 293-302.
- Jones LW, Eves ND, Courneya KS, Chiu BK, Baracos VE, Hanson J, Johnson L, Mackey JR. The effects of exercise training on antitumor efficacy of doxorubicin in MDA-MB-231 breast cancer xenografts. Clinical Cancer Research. 2005;11: 6695-8.
- Enoki T, Yoshida Y, Lally J, Hatta H, Bonen A. Testosterone increases lactate transport, monocarboxylate transporter (MCT) 1 and MCT4 in rat skeletal muscle. Journal of Physiology. 2006;577: 433-43.
- Zielinski MR, Muenchow M, Wallig MA, Horn PL, Woods JA. Exercise delays allogeneic tumor growth and reduces intratumoral inflammation and vascularization. Journal of Applied Physiology.2004;96: 2249-56.
- Murphy EA, Davis JM, Barrilleaux TL, McClellan JL, Steiner JL, Carmichael MD, Pena MM, Hebert JR, Green JE. Benefits of exercise training on breast cancer progression and inflammation in C3 (1) SV40Tag mice. Cytokine. 2011;55: 274-9.
- Verma VK, Singh V, Singh MP, Singh SM. Effect of physical exercise on tumor growth regulating factors of tumor microenvironment: implications in exercise-dependent tumor growth retardation. Immunopharmacology and Immunotoxicology. 2009;31: 274-82.
- Cuevas MJ, Almar M, García–Glez JC, García–López D, De Paz JA, Alvear–Ordenes I, González-Gallego J. Changes in oxidative stress markers and NF-κB activation induced by sprint exercise. Free Radical Research. 2005;39: 431-9.
- Gius D, Spitz DR. Redox signaling in cancer biology. Antioxidants and Redox Signaling. 2006;8: 1249-52.
- Vider J, Laaksonen DE, Kilk A, Atalay M, Lehtmaa J, Zilmer M, Sen CK. Physical Exercise Induces Activation of NF-κB in Human Peripheral Blood Lymphocytes. Antioxidants and Redox Signaling. 2011;3: 1131-7.
- Oliveras-Ferraros C, Cufí S, Vazquez-Martin A, Torres-Garcia VZ, Del Barco S, Martin-Castillo B, Menendez JA. Micro (mi) RNA expression profile of breast cancer epithelial cells treated with the anti-diabetic drug metformin: induction of the tumor suppressor miRNA let-7a and suppression of the TGFβ-induced oncomiR miRNA-181a. Cell Cycle. 2011;10: 1144-51.
- Barh D, Malhotra R, Ravi B, Sindhurani P. MicroRNA let-7: an emerging next-generation cancer therapeutic. Current Oncology. 2010;17: 70-80.
- Simon P, Fehrenbach E, Niess AM. Regulation of immediate early gene expression by exercise: short cuts for the adaptation of immune function. Exerc. Immunology Review. 2006;12: 112-31.
- Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, Heller R, Ellis LM, Karras J, Bromberg J, Pardoll D, Jove R, Yu H. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21: 2000-8.
- Bollrath J, Phesse TJ, von Burstin VA, Putoczki T, Bennecke M, Bateman T, Nebelsiek T, Lundgren-May T, Canli O, Schwitalla S, Matthews V, Schmid RM, Kirchner T, Arkan MC, Ernst M, Greten FR. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15: 91-102.








