Sayyed Mostafa Habibi-Khorassani*1, Mehdi Shahraki1, Malek Taher Maghsoodlou1, Eideh Mofarrah1, Elham Mofarrah2 and Sayyed Rasul Mousavi1
1Department of Chemistry, University of Sistan and Baluchestan, P. O. Box 98135-674, Zahedan, Iran, 2Department of Chemical Engineering, Amirkabir University of Technology (Tehran Polytechnic), Tehran, Iran
DOI : https://dx.doi.org/10.13005/bpj/799
Abstract
The hindered internal rotations around the partial carbon-carbon double bond (MeO2C↑=CPPh3), nitrogen-carbon (N↑-C-CH) and carbon-carbon single (MeO2C↑H-C-C=PPh3) bonds within dimethyl-2-(6-Aza uracil 1-yl)-3-(triphenylphosphoranylidene) butandioate has been experimentally studied by the dynamic 1H NMR spectroscopic in variable temperatures. The activation (∆H‡, ∆S‡, ∆G‡) and kinetic (kc, Ea.) parameters were calculated in a series of separate dynamic 1H NMR spectra. The energy of activation parameters obtained from classic, Eyring and Arrhenius methods were also compared for carbon-carbon double, this compound. The results from Eyring and Arrhenius plots were in good agreement, however, they were different with the classic method. Rotation around the carbon-carbon single bond ∆G‡ has low value than rotation around the carbon-carbon double bond and rotation around the carbon-carbon single bond.
Keywords
Dynamic 1H NMR; Activations Parameters; Internal Rotations; Ylide; Isomerism
Download this article as:| Copy the following to cite this article: Habibi-Khorassani S. M, Shahraki M, Maghsoodlou M. T, Mofarraha E, Mofarrah E, Mousavi S. R. Dynamic 1H NMR Study of the Hindered Internal Rotation in a Particular Biological Phosphorus Ylide Involving 6-Azauracil. Biomed Pharmacol J 2015;8(2) |
| Copy the following to cite this URL: Habibi-Khorassani S. M, Shahraki M, Maghsoodlou M. T, Mofarraha E, Mofarrah E, Mousavi S. R. Dynamic 1H NMR Study of the Hindered Internal Rotation in a Particular Biological Phosphorus Ylide Involving 6-Azauracil. Biomed Pharmacol J 2015;8(2). Available from: http://biomedpharmajournal.org/?p=3853 |
Introduction
Structure, properties, reactions mechanism and applications of P-ylides and their derivatives especially in the synthesis of naturally occurring products with pharmacological and biological activities are remarkable and important [1-9]. As phosphorus ylides from 6-Azauracil is not exception from the principle.
6-Azauracil or 6-Aza-2, 4-dihydroxypyrimidine is an inhibitor of enzymes that are involved in pyrimidine and purine biosynthesis, which leads to alterations in nucleotide pool levels in inside the body. Afterward the reduction of nucleotide levels by 6-azauracil can reduce transcription elongation [10]. The inhibition of GTP biosynthesis and IMP dehydrogenase activity in Saccharomyces cerevisiae by 6-azauracil has been studied [11]. 6-Aza-2, 4-dihydroxypyrimidine has been widely used in investigations on modulation of transcription, particularly in yeast model systems. In investigations of mutations in components of the RNA polymerase II transcription elongation machinery, 6-azauracil has been presented to induce transcription in wild type Saccharomyces cerevisiae of the PUR5 gene, which is one of four genes that encode IMPDH-related enzymes. It is worth noting mutants which are sensitive to 6-azauracil, for example mutants with a disrupted gene encoding elongation factor SII or containing amino acid substitutions in polymerase II subunits, are also faulty in PUR5 induction [12]. Other investigations on elongation-defective mutant yeast show that 6-azauracil treatment leads to diminished transcription of the GAL1 gene [13]. The addition of Saccharomyces cerevisiae mutants defective in the histone methyl transferase gene Set2 have been shown to have increased talent to 6-azauracil (treatment).
Some of phosphorus ylides exhibit dynamic 1H NMR effects that affords good information regarding the interchangeable process of rotational isomers that provide important kinetic data, and also it is useful tool when discussing the barriers separating two states that observable by NMR spectroscopy [14-17].
We wish to describe the dynamic 1H NMR effects on the phosphorus ylide 4 prepared of the reaction between triphenylphosphine 1 and dimethylacetylendicarboxylate 2 in the presence of 6-azauracil 3 that it’s synthesis has been reported previously [18]. The most common methods of determining activation parameters such as classic, Eyring and Arrhenius methods have been employed in the recent research work [19].
Chemicals and instruments
The chemicals used involving triphenylphosphine 1, dimethyl acetylendicarboxylate 2 and 6-azauracil 3 are all analytical grade, obtained from Fluka (Buchs, Switzerland). The extra pure (supplied by Merk, Darmstadt Germany) including ether and acetone-d6are used throughout the studies. The1HNMR spectra were recorded on a FTNMR 400.22 MHz-BRUKER-Avance III.
Results and Discussion
The 1H, 13C and 31P NMR spectra of ylide 4 confirmed the presence of two isomers (Fig .1) [21]
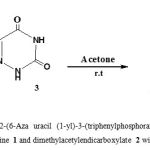 |
Figure 1: Synthesis of dimethyl-2-(6-Aza uracil (1-yl)-3-(triphenylphosphoranylidene) butandioate 4 from the reaction between triphenylphosphine 1 and dimethylacetylendicarboxylate 2 with1,5 carbazon 3 |
Moiety of the synthesized compound is strongly conjugated with the adjacent carbonyl group along with the three rotations around the partial carbon–carbon double bond (x), carbon–carbon single (y) and nitrogen–carbon single (z) bonds in the Z-4 and E-4 rotational isomers (Fig. 2).
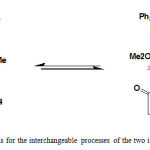 |
Figure 2: Three possible rotations for the interchangeable processes of the two isomers (Z– and E-) in ylide 4 |
The 1H NMR spectra of compound 4exhibited two doublets for methine proton (H–C–C=P) at and methyl groups δ = 5.351-5.402 and 3.622-3.652 ppm, for the minor and major geometrical isomers, respectively. All possible interchangeable process of rotational isomers for ylide 4 involving isomers I, II, III, IV, V and VI are shown in Fig. 3
![Figure. 3 Interchangeable process of rotational isomers for ylide 4 (1) Restricted rotational process (I, II, 343.15K) around the carbon–carbon double bond [entry a] (2)Restricted rotational process around carbon-carbon simple bond (I,III, 211.15 K) [entry b] and (II,IV,213.15 K) [entry c](3) Restricted rotational process (I ,v,241.15 K) [entry d] and (II,VI,243.15K) [entry d] around carbon–nitrogen simple bond.](https://biomedpharmajournal.org/wp-content/uploads/2016/01/Vol8_No2_Dyn_Sayy_fig3-150x150.jpg) |
Figure 3: Interchangeable process of rotational isomers for ylide 4 (1) Restricted rotational process (I, II, 343.15K) around the carbon–carbon double bond [entry a] (2)Restricted rotational process around carbon-carbon simple bond (I,III, 211.15 K) [entry b] and (II,IV,213.15 K) [entry c](3) Restricted rotational process (I ,v,241.15 K) [entry d] and (II,VI,243.15K) [entry d] around carbon–nitrogen simple bond. |
Dynamic effects for the Z-4 and E-4 rotational isomers as a result of the restricted rotational processes (Fig. 3, a, I and II) around the carbon–carbon double bond
The 1H NMR spectrum for the 4-Z and the 4-E (Fig. 3, a, I and II) in acetone at 50 º C (323.15 K) showed sharp triplet for the methine proton (H–C–C=P, 3JPH) .They are appreciably broadened at 60ºC (333.15 K) .This resonance coalescence occurred at near 70 ºC (343.15 K) which is relevant to restricted rotational process around carbon–carbon double bond. Another resonance coalescence is accrued at near 64 ºC (337.15 K) in relation to the methoxy group.
The classic method of determining activation energy parameters is through the determination of temperature at which NMR resonance of two exchanging species coalesce [19]. So, the coalescence temperature (Tc) and isotropic chemical shift (∆ν) is extracted in conjunction with the maximum peak separation in the low-temperature limit. Investigation of such behavior with respect to the ylide 4 coalescence temperature (Tc =343.15K) allowed us to also calculate the rotational energy barrier.
For this process, activation parameters (∆H‡, ∆S‡ and ∆G‡) and kinetic parameters (kc and Ea) are calculated by the classic method and tabulated[1] in Table 1.
[1]: ∆G‡ = aT{9.972 + log(Tc/∆ν) where a = 4.575 × 10-3 for unites of kcal/mol, at the coalescence temperature:kc= πΔυ/√2, ∆H‡ = ∆[log(kc/Tc)]/∆[1/Tc], ∆S‡ = (∆H‡ – ∆G‡)/T, Ea = ∆H‡ + nRTTable 1: The activation parameters derived from dynamic review ylide 4 around the carbon-carbon double bound (Entry a, Figure 3)
| Tc | δ | ∆ν | kc | ∆G‡ | ∆H‡ | ∆S‡ | Ea |
| K | ppm | Hz | s-1 | kcalmol-1 | kcalmol-1 | calmol-1K-1 | kcalmol-1 |
| 343.15 | 5.351-5.402 | 20.41 | 45.32 | 17.58 | |||
| 19.66 | 6.07 | 20.34 | |||||
| 337.15 | 3.622-3.652 | 12.01 | 26.66 | 17.615 |
Due to the isotropic chemical shifts of the exchanging species are dependent on temperature,
So accurately determining of ∆ν is often difficult and thus estimation of the activation energy barrier (∆G‡) has a large error.
Therefore, to complete analysis and reducing the errors, the determining of activation parameters are performed by Arrhenius plot and Eyring plot methods. With respect to the average value of
[1]: ∆G‡ = aT{9.972 + log(Tc/∆ν) where a = 4.575 × 10-3 for unites of kcal/mol, at the coalescence temperature:kc= πΔυ/√2, ∆H‡ = ∆[log(kc/Tc)]/∆[1/Tc], ∆S‡ = (∆H‡ – ∆G‡)/T, Ea = ∆H‡ + nRTBest results for ∆S‡ will be obtained by drawing a new plot (Figure 4B), where ∆S‡ (that is sensible sensor) can be found from the slope. The activation parameters are accumulated in Table 3 for comparing the derived data with the previous data. By picking a temperature (T = 343.15 K) and comparing the magnitudes of ∆H‡ and T∆S‡, we can decide if the step is enthalpy-controlled or entropy-controlled. For rotation around the carbon-carbon double bond ∆H‡ = 15.82 kcalmol-1 and T∆S‡ = -0.0006 kcalmol-1, so enthalpy and entropy are both important and rotation around the double bond is controlled by enthalpy and entropy. This agrees with this process. Because in the rotation around the double bond, leaving solution will involve breaking double bond with solvent (enthalpy) and losing the freedom of the molecule (entropy).
The standard errors of the Eyring and Arrehnius equations for activation parameters are also calculated[1].This correlation has been mentioned elsewhere [20-22]. The values of activation energy and activation parameters (∆G‡, ∆H‡, ∆S‡) obtained from three methods are reported in Table 3.
Table 2: Determination ∆G‡, lnk/T and lnk for rotation around carbon-carbon double bond (Entry a, Fig. 3)
| Tc
K |
1/T | ∆δ
ppm |
∆ν
Hz |
∆G‡
kcal/mol |
Lnk/T | Lnk | T×Lnk/T | |||||||||
|
293.15 |
0.00341 |
5.293-5.337 |
17.61 |
15.10 |
-3.40 |
2.27 |
-996.71 |
|||||||||
| 303.15 | 0.00329 | 5.313-5.360 | 18.81 | 15.58 | -2.51 | 3.20 | -760.906 | |||||||||
| 313.15 | 0.00319 | 5.334-5.383 | 19.61 | 16.07 | -1.67 | 4.08 | -522.96 | |||||||||
| 323.15 | 0.00309 | 5.351-5.402 | 20.41 | 16.55 | -0.88 | 4.89 | -285.34 | |||||||||
| Average15.82 | ||||||||||||||||
[1]: ln(k/T)=23.76+ ∆S‡/R – ∆H‡/RT, slope = – ∆H‡/R and intercept point= ∆S‡/R +23.76, ∆G‡ = ∆H‡ – T∆S‡, Ea = ∆H‡ + RT [1]: lnk = lnA – (Ea/R)(1/T), slope = –Ea/R, ∆H‡ = Ea– RT [1]: σ(∆S‡) = (1/Tav)σ(∆H‡) where Tav= is the center of the temperature range studied, σ(∆S‡) = σ(∆H‡×0.0034 K-1)
 |
Figure 4A: An Eyring plot of ln (k/T) versus 1/T |
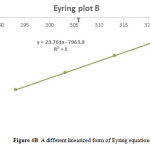 |
Figure 4B: A different linearized form of Eyring equation |
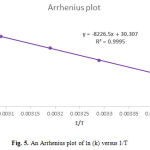 |
Figure 5: An Arrhenius plot of ln (k) versus 1/T |
Table 3: Comparing the obtained results from the three methods (Classic, Eyring and Arrhenius) for the restricted rotation around the carbon-carbon double bond in the isomer Z-4 and E-4 [entry a]
| Methods | ∆G‡
(kcalmol-1) |
∆H‡
(kcalmol-1) |
∆S‡
(calmol-1K-1) |
Ea
(kcalmol-1) |
||
| Classic | 17.58 | 19.66 | 6.07 | 20.34 | ||
| Eyring A | 15.86 | 15.74 ±0.32 | -0.33 ± 0.001 | 16.42±0.32 | ||
| Eyring B | 15.83 | 15.83±0.32 | 0.00199± 0.001 | 16.51±0.32 | ||
| Arrhenius | 15.78 | 15.66 ±0.32 | -0.33 ±0.001 | 16.35±0.32 | ||
Comparing calculated results (Table 3) using three methods indicate that the results from Eyring and Arrhenius plots were in good agreement, however, were different with the classic method
Dynamic effect for the 4-Z and 4-E rotational isomers as a result of restricted rotational processes (Fig.3, Z entry b,I and III ,E,entry c, II and IV ) around the carbon–carbon simple bond
Herein, when temperature is reduced down lower than ambient temperature, the 1H NMR spectrum for the 4– Z isomer (Fig. 3, b, I and III) in acetone-d6 shows a resonance arising from methoxy proton (3. 625-3.640 ppm) which is appreciably broadened comparison with a corresponding singlet which is measured at 10 ºC ( 283.15K) . The resonance coalescence occurs at approximately -62 ºC (211.15 K) and appears as a singlet resonance at -90 ºC (183.15K), the lowest temperature investigated, which is relevant to the restricted rotational process around the carbon–carbon single bond.
Also, when temperature is reduced down lower than ambient temperature, the 1H NMR spectrum for the 4–E isomer (Fig. 3, c, II and IV) in acetone-d6 shows a resonance arising from methoxy group ( 3.640-3.765 ppm) which is appreciably broadened comparison with a corresponding a singlet which is measured at 10ºC ( 283.15K). The resonance coalescence occurs at approximately -60 ºC (213.15 K) and appears as a singlet resonance at -90 ºC (183.15K), the lowest temperature investigated, which is relevant to the restricted rotational process around the carbon–carbon single bond. For this process, ∆G‡, kc are calculated and presented in Table 4.
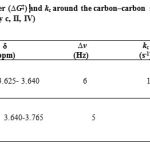 |
Table 4: Activation energy barrier (∆G‡) and kc around the carbon–carbon simple bond for isomers Z and E (4-Z Entry b, I, III and 4-E Entry c, II, IV) |
Dynamic effect for the 4-Z and 4-E rotational isomers as a result of restricted rotational processes (Fig. 3, Z, entry d, I and V, E, entry e, II and VI) around the nitrogen–carbon (N -C-CH) bond
when temperature is reduced down lower than ambient temperature, the 1H NMR spectrum result for the 4– Z isomer (Fig. 3, d, I and V) in acetone-d6 shows a resonance arising from methoxy ( 2.996-3.004 ppm) that is appreciably broadened in comparison with a corresponding doublet that was measured at 20 ºC (293.15 K). This resonance coalescence occurred at approximately -32 ºC (241.15 K) which is relevant to restricted rotational process around nitrogen–carbon single bond and appears as a doublet resonance at -90 ºC (183.15K), the lowest temperature investigated, which is relevant to the restricted rotational process around the nitrogen–carbon single bond.
Another resonance coalescence is accrued at near -28 ºC (245.15 K) in relation to the methine proton (H–C–C=P, 3JPH).
Also, the 1H NMR spectrum for the 4-E (Fig. 3, entry e, II and VI) in acetone-d6 shows a resonance arising from methoxy (3.284- 3.384.15 K ppm) that is appreciably broadened in comparison with a corresponding doublet that was measured at 20 ºC (283.15 K) for isomer E. This resonance coalescence occurred at approximately -30ºC(243.15K) which is relevant to restricted rotational process around nitrogen–carbon single bond and appears as a doublet resonance at -90 ºC (183.15K), the lowest temperature investigated, which is relevant to the restricted rotational process around the nitrogen–carbon single bond. Another resonance coalescence is accrued at near-26ºC (247.15 K) in relation to the methine proton (H–C–C=P, 3JPH). For this process, the activation parameters involving ∆G‡, ∆H‡ and ∆S‡, and kinetic parameters (kc and Ea) obtained using the classic method for 4– Z and 4-E isomers is reported in Table 5.
Table 5: Activation parameters of phosphorus ylide4for rotation around the nitrogen- carbon single bond in the Z-4 and 4-E isomer, (4-Z, entry d, I, V, and 4-E, entry e, II, VI) according to classic method
| isomers Tc (K) | δ (ppm) | ∆ν (Hz) | kc (s-1) | ∆G‡ (kcalmol-1) | ∆H‡ (kcalmol-1) | ∆S‡ (calmol-1K-1) |
Ea (kcalmol-1) |
| Z 241.15 | 2.996-3.004 | 3.2 | 7.11 | 13.07 | |||
| 12.63 | -1.82 | 13.11 | |||||
| Z 245.15 | 5.08-5.205 | 5 | 11.11 | 13.08 | |||
| E 243.15 | 3.284-3.384 | 4 | 8.88 | 13.08 | |||
| 11.62 | -5.99 | 12.11 | |||||
| E 247.15 | 5.205-5.22 | 6 | 13.33 | 13.10 |
Conclusion
We reported dynamic 1H NMR effects around the three chosen bonds within a synthesized ylide.
- The three most common methods of determining activation parameters are used to find out which of the methods deviate from each other for carbon-carbon double bond. The results obtained from the Eyring and Arrhenius methods were consistent, however, were different with the classic method.
- Activation parameters were calculated using classic method (single point) for nitrogen–carbon bond for Z isomer and E isomer. The Eyring and Arrhenius method did not use in these rotations because required data were not available.
- The value of activation barrier energy (∆G‡) and kc were calculated for carbon–carbon simple bond for Z isomer and E isomer. The classic method (single point), Eyring and Arrhenius method did not use in these rotations because required data were not available.
- In this study, due to the strong conjugation with the adjacent carbonyl group, the value of activation barrier energy (∆G‡) of the restricted rotation around the partial carbon-carbon double bond (MeO2C=CPPh3) was larger than the nitrogen-carbon (N-C-CH) and carbon-carbon (MeO2CH-C-C=PPh3) single bonds. As a case study, because of the lone pair inversion of nitrogen atom in ring, rotation around the carbon-nitrogen single bond needs a barrier rotational energy more than the carbon-carbon single bond.
- Rotation around the carbon-carbon double bond ∆G‡ has a more positive value. So high value of ∆G‡ indicates the rate of this process is slow at room temperature. At higher temperature it is fast and spontaneous.
- Rotation around the carbon-carbon single bond ∆G‡ has low value (less position value), so rotation around the carbon-carbon single bond is a fast process and spontaneous at room temperature, observation of two conformers (I, II or III and IV) on the basis of this rotation at low temperature is a good evidence for the process that is not spontaneous under this condition.
Acknowledgement
We gratefully acknowledge financial support from the Research Council of the University of Sistan and Baluchestan.
References
- M.Tawkir, S.A. Iqbal, B. Krishan, I. Zaafarany, Orient. J. Chem. 2011, 27(2), 603-609
- N.Hazeri, S. M. Habibi-Khorassani, M. T. Maghsoodlou, G. Marandi, M. Nassiri, G. J. Shahzadeh, Chem. Res. 2006 (3), 215-217
- M. T Maghsoodlou, S. M Habibi-Khorassani, N.Hazeri, M.Nassiri, R.Kakaei, G. Marandi, Phosphorus, Sulfur Silicon Relat. Elem. 2006, 181(3) 553-560.
- B. E .Maryanoff, A. B .Rietz, Chem. Rev. 1989, 89 (4) , 863-927.
- L. An-Hu, D. Li-Xin, V. Aggrwal, chem. Rev. 1997, 97(6), 2341-2372.
- O.I. Kolodiazhnyi, Russ. Chem. Rev. 1997, 66(3),225-254.
- K.M. Pietrusiewiz, M. Zablocka, Chem. Rev. 1994(5), 94, 1375-1411.
- S. M . Habibi-Khorassani , M. T Maghsoodlou ,A. Ebrahimi ,Y.Ghalandarzehi. F.Ghodsi,O. Asher, M. J. Iranian Chem. Soc. 2012, 28(3), 1153-1169.
- K.M. Pietrusiewiz, M. Zablocka, Chem. Rev. 1994(5), 94, 1375-1411.
- F. A. L. Anet. R. Anet, F. A. Cotton, L.M. Jackman, Eds., Academic Press: New York, 1979, 8.
- E. Mofarrah, S. M. Habibi-Khorassani, M. T. Maghsoodlou, M. Shahraki, Applied Magnetic Resonance, 2015. 10.1007/s00723-015-0703-2.
- S.M. Habibi-Khorassani, M.T. Maghsoodlou, A. Ebrahimi, M. Mohammadi, M. Shahraki, E. Aghdaei, J. Phys. Org. Chem. 2012, 25(12), 1328-1335.
- S.M. Habibi-Khorassani, M. Shahraki, M.T. Maghsoodlou, S. Erfani, Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2015, 145, 410–416.
- M. Shahraki, S.M. Habibi-Khorassani, A. Ebrahimi, M. Maghsoodlou, Y. Ghalandarzehi, Struct Chem. 2012, 24, 623-635.
- Exinger, F., and Lacroute, F., 6-Azauracil inhibition of GTP biosynthesis in Saccharomyces cerevisiae. Curr. Genet, 1992, 22(1), 9-11
- Shaw, R. J., and Reines, D., Saccharomyces cerevisiae transcription elongation mutants are defective in PUR5 induction in response to nucleotide depletion. Mol. Cell. Biol., 2000, 20(20), 7427-7437
- Wind-Rotolo, M., and Reines, D., Analysis of gene induction and arrest site transcription in yeast with mutations in the transcription elongation machinery. J. Biol. Chem, 2001, 276(15), 11531-11538
- Li, J., et al., Association of the Histone Methyltransferase Set2 with RNA polymerase II Plays a Role in Transcription Elongation. J. Biol. Chem, 2002, 277(51), 49383-49388
- S.M. Habibi-Khorassani, M.T. Maghsoodlou, N. Hazeri, R. Heydari, K. Bagherpour, M. Rostamtamzadeh, H. Najafi, Phosphorus, Sulfur Silicon Relat. Elem. 2010, 186(1) 21-30.
- K.D. Zimmer, R. Shoemaker, R.R. Rumi ski, Inorganic Chemical Acta. 2006, 359(5), 1478-1484.
- G. Lente, I. Fabian, A.J. Poe, New J. Chem. 2005, 29(6), 759-760.
- J.H. Espenson, McGraw-Hill, New York, 1995, 2,158.
- A. J. Poe., ed. M.V.Twigg, Plenum Press.1994,8,10,220.








