I Made Sukadana and Sri Rahayu Santi
Chemistry Department Faculty of Mathematic and Natural Sciences Udayana University
DOI : https://dx.doi.org/10.13005/bpj/797
Abstract
Gayam seed (Inocarpus fagiferus Fosb) is empirically has an ability to reduce oxidative stress diseases such as atherosclerosis. This study aims to prove the antioxidant compounds such as linoleic acid, ethyl linoleic, ethyl oleic, and homopterocarpine in ethanol extract of gayam seed (Inocarpus fagiferus Fosb) to prevent atherosclerosis through increase SOD-3 expression and decrease of MDA concentration plasma blood of Wistar rat with high cholesterol diet for 16 weeks. This was an experimental study with randomized posttest only control group design. The samples were 25 Wistar rat, randomized into 5 treatment groups, i.e. group P0 (negative control), group P1 (positive control, feed high cholesterol diet), group P2 (high cholesterol diet and ethanol extract in dose of 50 mg/kg bw), group P3 (high cholesterol diet and ethanol extract in dose of 100 mg/kg bw), and group P4 (high cholesterol diet and ethanol extract in dose of 150 mg/kg bw). After 16 weeks treatment, blood of the rats were driven for MDA and all rats were then euthanasia to obtain their aorta for immunohistochemistry analyzed to give expression of SOD-3 data. All of data was analyzed by Anova to obtain the treatment different toward control by statistically with significance at a =0.05. The results showed that ethanol extracts of gayam seed (Inocarpus fagiferus Fosb) in doses of 50-150 mg/kg bw increase the expression of positive SOD-3 aorta endhotel cell and decrease the concentration of MDA significantly (p<0.05). The expression of positive SOD-3 aorta endhotel cell is marker endogenous antioxidant, therefore, the antioxidant compounds in ethanol extract of gayam seed can to prevent atherosclerosis disease through of SOD inducer mechanism.
Keywords
Inocarpus fagiferus Fosb; antioxidants; SOD-3, MDA; atherosclerosis
Download this article as:| Copy the following to cite this article: Sukadana I. M, Santi S. R. Antioxidant Compounds of Gayam Seed (Inocarpus Fagiferus Fosb) to Prevent Atherosclerosis in Wistar Rat with High Cholesterol Diet . Biomed Pharmacol J 2015;8(2) |
| Copy the following to cite this URL: Sukadana I. M, Santi S. R. Antioxidant Compounds of Gayam Seed (Inocarpus Fagiferus Fosb) to Prevent Atherosclerosis in Wistar Rat with High Cholesterol Diet. Biomed Pharmacol J 2015;8(2). Available from: http://biomedpharmajournal.org/?p=3186 |
Introduction
Nowadays the case of heart disease (cardiovascular) is increasing in all parts of the world, including in Indonesia. One of the major risk factors of cardiovascular disease and its complications is atherosclerosis.
Atherosclerotic disease is one of the major sources of morbidity and mortality of the population in developed and developing countries (Stocker and John, 2004; Nageswara et al., 2005). Approximately 39% of deaths in the UK population and nearly one million per year in the US are caused by atherosclerosis and its complications (Lefkowitz and Willerson, 2001). Elena and Klaus (2007) in citing the WHO Health Report 2003, stated that about 20% of cases, or 16.7 million people worldwide deaths per year are caused by atherosclerosis risk of stroke and coronary heart disease (ischemic heart disease / IHD). It is estimated that by 2020, the disease is still the leading cause of death and health problems in the world and in Indonesia. In Indonesia, the recorded number of deaths caused by heart disease and atherosclerosis is increasing every year.
Atherosclerosis is a disease characterized by oxidative stress thickening of the arterial wall due to the proliferation of certain cells that affects the blood flow resistance. One of the main risk factors are hypercholesterolemia (Stocker and John, 2004; Ragavendra and Christopher, 2012), a condition in which the levels of cholesterol in the blood is very high, which is correlated with the condition of the provision of a high-fat diet. High levels of cholesterol in the blood will increase the produces of reactive oxygen species (ROS), especially superoxide anion, an oxidative stress molecule. ROS can oxidize LDL-cholest molecules to their oxidized form of LDL (ox-LDL), which lead to endothelial cells (endothelial dysfunction) damage in the walls of arteries. ROS molecules also cause oxidation of polyunsaturated fatty acids from LDL-cholest to produce an aldehyde molecule that is malondialdehida (MDA), where the measurement of MDA can be used as an indirect index of oxidative damage caused by peroxidation of lipids (Stefan et al., 1996; Ahmed, 2001; Han et al., 2002; Stocker and John, 2004).
To prevent LDL-cholest oxidation process, the body has an endogenous antioxidant, one of which is the superoxide dismutase (SOD) in the form of SOD-3 which it catalyze the reaction that is changing the anion radical superoxide (O2) into a relatively stable molecule that is hydrogen peroxide (H2O2) and oxygen (O2). The ability of endogenous antioxidants prevents oxidation of LDL-cholest depends on the number of SOD in the body that are expressed in a cell. If the body have less the number of SOD so the body requires consumed exogenous antioxidant that can source of nature.
Gayam (Inocarpus fagiferus Fosb) or in Bali known as gatep is one of the herbs, whose seeds are ethnobotanycally be used for anti-inflammatory and antioxidant, and have various medicinal properties (Heyne, 1987; Segatri, 1995; Pauku , 2006). The result of study indicates that the ethanol extract of gayam seed contains unsaturated fatty acid or esters of unsaturated fatty acids such as linoleic acid, ethyl linoleic, ethyl oleic, and homopterocarpine, a flavonoid derivative compound. These compounds evidenced can increase activity of antioxidant endogen SOD and decrease the levels of lipid profile i.e total cholesterol, triglyceride, and LDL cholesterol, except the HDL cholesterol in blood plasm of Wistar rat so it are potential to prevent atherosclerosis disease (Sukadana et al., 2015).
Therefore the ethanol extract of gayam seed can increase SOD activity so it is crucial to investigate their effect toward expression of SOD-3 in aorta endothel and their potent as an antioxidant agent to prevent oxidation of polyunsaturated fatty acids from LDL-cholest. In this paper the level of MDA in blood plasm and expression of SOD-3 in aorta endhotel are described.
Materials and Methods
Materials
Materials used in this research are: gayam seed (Inocarpus fagiferus Fosb) seed, obtained from Tabanan Bali and taxonomycally identified by LIPI’s Kebun Raya “Eka Karya” Bali. Chemicals used are ethanol (p.a and technical) and aquadest. Animal used is Wistar rat and with ethical clearance No. 0144/KE-PH/IX/2013 dated: 4 September 2013. Chemicals that used to immunohistochemical analysis are formalin 10%, buffered citrat 0,1 M (pH. 7,4), kit LSAB (Dako, Denmark) and antibody primer Rabbit Anti-SOD-3 Polyclonal Antibody (Bioss, Cat. bs-3895R), and another chemicals from Sigma-Aldrich (USA) as metanol p.a, thripsine 0,25%, H2O2 3%, formalin buffered phosphate 10%, alcohol 70%, 90%, 96%, 100%, xylene, paraphine FBS 5%, Biotinylated Goat Anti-Polyvalent, Streptavidin peroxidase, aquabidest, hematoxylin Gill, and buffer tri sodium citrat. Chemicals to MDA analysis are TBARS Assay (ParameterTM, Cat. KGE013) its content TBA Reagent, TBARS Standart, TBARS Acid Reagent, sulphate acid 0,05 N, ethanol 96%, dietilether, and n-buthanol. Other material were Whatman No.1, ketamine and xylazine for anesthesia, blood plasm, standard feed BR1 CP511B, high fat diet feed consist of mixture BR1 CP511B and other materials with high cholesterol.
Equipments
Equipments used include a set of glass wares, extractor, analytical balance, blender, knife, rotary vacuum evaporator, desicators, and test tubes, volumetric pipettes, rat cage, syringe, micro haematocrit tubes (Cat. 7493 21), EDTA Eppendorf tubes, sentrifuge 2-6E Sartorius, microtom rotary (Jung Histocut Leica 820), microscope binoculer Olympus CX41 with camera Olympus DP12, photographed with an Optilab Pro (Miconos, Indonesia) camera. Each preparation was photographed 5 times by in JPEG format using Optilab Raster Image Viewer 1.0 and 2.1 software (Miconos, Indonesia). microscope slide polylysine, coverslip, and UV-vis spectrophotometer Varian DMS 80.
Procedure
Extraction of Gayam Seed and Apply to Wistar Rat
About 5.0 kg dried powder of gayam (Inocarpus fagiferus Fosb) seed was macerated using 20 L ethanol 96% (EtOH). Maceration process was conducted 3 times at 24 hours each. The ethanol extract was evaporated using rotary vacuum evaporator to obtain 87.272 g concentrated EtOH extract. It was then was applied to animal model on Wistar rat in dose 50, 100, and 150 mg/kg bw rat every days to prove the potency of concentrated EtOH extract of gayam seed to prevent atherosclerosis through increase of expression SOD-3 and decrease of MDA level using to randomized posttest only control group design with 5 groups.
Group P0: negative control (rat group with feed standard)
Group P1: positive control (rat group with feed high fat diet)
Group P2: rat group with feed high fat diet + ethanol extract dose 50 mg/kg bw
Group P3: rat group with feed high fat diet + ethanol extract dose 100 mg/kg bw
Group P4: rat group with feed high fat diet + ethanol extract dose 150 mg/kg bw
The experiment was conducted for four months then the blood and aorta endhotel of each rat groups were analyzed for their MDA levels and expression SOD-3. These data were analysed using one way Anova (SPSS version 19.0 for Windows). Descriptive analysis to provide an overview of the characteristics data. Test for normality with Shapiro-Wilk test, and homogeneity with Levene Test. Comparison test conducted by One Way Anova and to see the difference between groups was followed by Post Hoc Test Tamhane. All tests with significance level of 5% (a = 0.05).
Results and Discussion
Weight of Wistar Rat
The everage weight of all rat groups for 16 weeks of treatment were 209-223 g as presented in Figure 1.
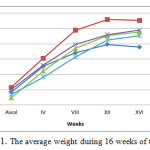 |
Figure 1: The average weight during 16 weeks of treatment |
Figure Description
P0 is a group of Wistar rats fed with standard (negative control), P1 is a group of Wistar rats fed with high cholesterol (positive control), P2 is a group of Wistar rats fed with high cholesterol and 50 mg / kg bw ethanol extracts of gayam seed, P3 is a group of Wistar rats fed with high cholesterol and 100 mg / kg bw ethanol extracts of gayam seed, P4 is a group of Wistar rats fed with high cholesterol and 150 mg / kg bw ethanol extracts of gayam seed.
MDA Level of Blood Plasm
The average plasma MDA of the five groups of rat after 16 weeks of treatment are presented in Figure 2. as follows:
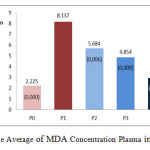 |
Figure 2: The Average of MDA Concentration Plasma in All Groups |
Figure Description
P0 is a group of Wistar rats fed with standard (negative control), P1 is a group of Wistar rats fed with high cholesterol (positive control), P2 is a group of Wistar rats fed with high cholesterol and 50 mg / kg bw ethanol extracts of gayam seed, P3 is a group of Wistar rats fed with high cholesterol and 100 mg / kg bw ethanol extracts of gayam seed, P4 is a group of Wistar rats fed with high cholesterol and 150 mg / kg bw ethanol extracts of gayam seed. P2, P3, and P4 decreased MDA concentrations significantly compared to P1 (p<0.05). The result of the average difference between groups: P0 vs. P1, p<0.05; P2 vs P1, p<0.05; P3 vs P1, p<0.05; P4 vs P1, p<0.05; P2 vs P3, p>0.05; P2 vs P4, p<0.05; P3 vs P4, p<0.05.
The high intake of cholesterol (hypercholesterolemia) increased MDA levels significantly in group P1 compared to standard groups (P0), P2, P3, and P4 (p<0.05), as shown in Figure 2. Hypercholesterolemia conditions as in P1 will trigger high mm-LDL formation (lipid peroxide) in the intima layer (subendothelial), which migrates mm-LDL to endothelial cells causes inactivation of nitric oxide (NO) in the endothelial cells that causes endothelial disfunction. The endothelial cells that undergo lipid peroxidation will result in high levels of oxidation product compounds such as malondialdehyde (MDA).
Increase of MDA levels in groups of rat given a high-cholesterol is a sign of a state of oxidative stress due to hypercholesterolemia (Singhania et al., 2008; Abdelhalim, 2010). Hypercholesterolemia will increase the production of reactive oxygen species (ROS) (Cai and Harrison, 2000; Madamanchi et al., 2004). Increase of ROS, especially superoxide anion (×O2–) will trigger a chain reaction in LDL lipids thus increases the formation of lipid peroxides (mm-LDL) on endothelial cells and lipid damage which in turn causes cell disfunction and increase the MDA as the reaction product of lipid peroxidation (Stefan et al., 1996; Ahmed, 2001; Han et al., 2002; Stocker and John, 2004). The results are consistent with the by research Han et al. (2007) and Chen et al. (2011) in rat given a high-fat feeding resulted in conditions of hypercholesterolemia and increasing levels of plasma MDA.
Ethanol extract of gayam seed is proven to reduce oxidative stress compounds that contain unsaturated fatty acid or esters of unsaturated fatty acids such as linoleic acid, ethyl linoleic, ethyl oleic, and homopterocarpine, a flavonoid derivative compound (Sukadana et al., 2015). These compounds are antioxidants because their molecules contain conjugated double bonds and hydroxyl groups (-OH) or methoxy (-OCH3) which allow the transfer of electrons, the release of H + or -CH3 molecules to form a radical anion (e) which will be donated to or reacts with ROS (superoxide anion) to form a relatively stable radicals through the mechanism of free-radical scavenging. Therefore, ROS is captured by the radical form of the antioxidant compounds, the lipids in LDL lipid peroxide is protected so that the compounds formed decreases and peroxide products such as MDA also decrease.
Expression of SOD-3 Aorta Endhotel
Figures 3 and 4 describe the average and an overview of positive expression of SOD-3 rat aortic endothelial cells based on immunohistochemical analysis using Rabbit Anti-SOD-3 polyclonal antibody cell marked brown on the edges or the cell nucleus, whereas SOD-3 expression negatively characterized by endothelial cells are colored blue on the edges or the cell nucleus.
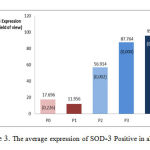 |
Figure 3: The average expression of SOD–3 Positive in all group |
Figure Description
P0 is a group of Wistar rats fed with standard (negative control), P1 is a group of Wistar rats fed with high cholesterol (positive control), P2 is a group of Wistar rats fed with high cholesterol and 50 mg / kg bw ethanol extracts of gayam seed, P3 is a group of Wistar rats fed with high cholesterol and 100 mg / kg bw ethanol extracts of gayam seed, P4 is a group of Wistar rats fed with high cholesterol and 150 mg / kg bw ethanol extracts of gayam seed. P2, P3, and P4 significant increase in the expression compared to P1 (p <0.05). The result of the average difference between groups: P0 vs. P1, p> 0.05; P2 vs P1, p< 0.05; P3 vs P1, p <0.05; P4 vs P1, p <0.05; P2 vs P3, p< 0.05; P2 vs P4, p< 0.05; P3 vs P4, p> 0.05.
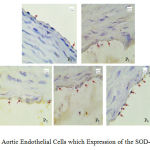 |
Figure 4: Aortic Endothelial Cells which Expression of the SOD-3 Positive |
In the group of rat fed with high cholesterol (P1) occurs oxidative stress impact on increasing the formation of ROS, especially superoxide anion (×O2–) which is unstable and reactive. Endogenous antioxidants SOD reacts with ROS as a response of of the body to prevent the formation of free radical molecules resulting in a decrease in the expression of new SOD (Fukai et al., 2002; Faraci and Didion, 2004). The mechanism is an endogenous antioxidation of SOD by breaking the chain reactions that convert the unstable and reactive superoxide anion into a more stable form of hydrogen peroxide (H2O2), oxygen (O2), and water (H2O), as shown in the following reaction:
2H+ + 2 ×O2– ¾¾¾ → H2O2 + O2
Anion superoxide
As long as the body provides sufficient endogen antioxidants SOD to neutralize the reactivity of superoxide anion radical (×O2–), then the effect of free radicals superoxide anion has no effect on the cell or not cause cell dysfunction.
As shown in Figures 3 and 4, the groups of rat in P2, P3, and P4 have significantly higher expression of SOD-3-positive (p<0.05) when compared with positive controls P1. In other words, the larger the ethanol extract of gayam dose given, the higher the expression of SOD-3-positive.
Based on the description above it can be concluded that the positive expression of SOD-3 increased significantly in the groups of rats were given high-cholesterol feed and ethanol extracts gayam seed in doses of 50 mg/kg bw (treatment 1, P2), 100 mg/kg bw (treatment 2, P3), and 150 mg/kg bw (treatment 3, P4) compared to the group of rat that were given high-cholesterol feed (P1) (p<0.05). The increases in the expression of SOD-3 positive are due to the antioxidant compounds contained in the ethanol extract of gayam seeds such as linoleic acid, ethyl linoleic, ethyl oleic, and homopterocarpine which can inhibit the oxidation reaction by the way it reacts with superoxide anion (×O2–) to form relatively stable molecules. Decrease in the number of superoxide anion radicals prevent new free radicals formation, and the formation of molecules which are relatively more stable, which play an important role in inducing endogenous antioxidant SOD (SOD inducer) so that its expression is increased.
Further Research
In vivo experiment of ethanol extract to obtain its potential as antiatherosclerosis with relevant variables including ICAM-1, VCAM-1, SOD-2, etc.
Acknowledgements
We would like to express our gratitude to Dikti that gave fund through Hibah Bersaing and Udayana University that facilite our research.
References
- Abdelhalim, M.A.K. The Potential Influnece of High Cholesterol Diet-induced Oxidative Stress on Composition and Properties of Red Bloods Cells in Rabbit. African Journal of Microbiology Research, 2010; 4(9): 836-43.
- Ahmed, E. Immune Mechanism in Atherosclerosis. Dissertation, ISSBN: 91-628-4612-4, Konferensrummet, Centrum for Molekular Medicin, Karolinska Sjukhuset, 2001.
- Cai, H., Harrison, D.G. Endothelial Dysfunction in Cardiovascular Disease: The Role of Oxidant Stress. Circulation Research, 2000; 87: 848-4.
- Chen, W.P., Mao, T.J., Fan, L., Zhou, Y.H., Yu, J., Jin, Y., Hou, P.C. Effect of Purple Sweet Potato on Lipid Metabolism and Oxidative Stress in Hyperlipidemic Rats. Chinese, 2011; 40(4): 360-4.
- Elena Galkina and Klaus Ley. Vasculas Adhesion Molecules in Atherosclerosis. Arteriosler Thromb Vasc Biol, 2007; 27: 2292-2301.
- Faraci, F.M., and Didion, S.P. Vascular Protection Superoxide Dismutase Isoforms in the Vessel Wall. Arterioscler Thromb Vasc Biol, 2004; 24: 1367-73.
- Fukai, T., Folz, R.J., Landmesser, U., Harrison, D.G. Extracellular Superoxide Dismustase and Cardiovascular Disease. Cardiovasc Res, 2002; 55(2): 239 –49.
- Han, S.N., Leka, L.S., Lichtenstein, A.H., Ausman, L.M., Schaefer, E.J., and Meydani, S.N. Effect of Hydrogenated and Saturated, Relative to Polyunsaturated, Fat on Immune and Inflammatory Responses os adults with Moderate Hypercholesterolemia. Journal of Lipid Reasearh, 2002; 43(3): 445-52.
- Han, X., Shen, T.,and Lou, H. Dietary Polyphenol and Their Biological Significance. Int.J.Mol Sci, 2007; 8: 950-88.
- Heyne, K. Tumbuhan Berguna Indonesia III, terjemahan: Badan Penelitian dan Pengembangan Kehutanan. Jakarta: Yayasan Sarana Wana Jaya, 1987; pp.
- Lefkowitz, R.J., and Willerson, J.T. Prospects for Cardiovascular Research. JAMA, 2001; 285: 581-7.
- Madamanchi, N.R., Vendrov, A., Runge, M.S. Oxidative Stress and Vascular Disease. Arterioscler. Throm Vasc Biol, 2004; 25(1): 29-38.
- Nageswara, R.M., Aleksandr, V., Marschall, S.R. Oxidative Stress and Vascular Disease. Arterioscler Thromb Vasc Biol, 2005; 25: 29-38.
- Pauku, R.L. Inocarpus fagifer (Tahitian chestnut), Species Profiles for Pasific Island Agroforestry, 1987; Available from: URL: www.traditionaltree.org, acsses 17 Nopember 2011
- Ragavendra, R.B., Christopher, P.C. Dyslipidemia, Oxford University Press, New York, 2012.
- Segatri, P. Taru Permana Khasiat Tanam-tanaman untuk obat Tradisional. Denpasar: Upada Sastra, 1995; pp.
- Singhania, N., Puri, D, Madhu, S.V., and Sharma, S.B. Assesment of Oxidative Stress and Endothelial Dysfunction in Asia Indians with Type 2 Diabetes mellitus with and Without Macroangiopathy. QJM, 2008; 101(6):449-55.
- Stefan, J., Mikko, P.SA., Bengt, K., and Jan-Nilsson. Human Monocytes/ Macrophages Release TNF-a in Response to Ox-LDL. Arteriosclerosis, Thrombosis, and Vascular Biology, 1996; 16: 1573-9.
- Stocker, R., and John, F.K.JR. Role of Oxidative Modifications in Atherosclerosis. Physiol.Rev, 2004; 84: 1381-478.
- Sukadana, I.M., Putra Manuaba, I.B., Wita, I.W., Sutirta Yasa, I.W.P., Santi, S.R. Antioxidant Compouds of Gayam Seed (Inocarpus fagiferus Fosb) to Prevent Atherosclerosis through Increase of SOD Activirty and Improvement of Lipid profile on Wistar Rat. J.Biol.Chem.Research, 2015; 32(1): 28-35.








