Vladimir S. Avdeenko1, Pavel. V Rodin1, Alexei A. Volkov1, Alexey V. Molshanov1, Kirill V. Plemyashov2, Anna Potapova3
1Saratov State Agrarian University, Saratov, Russian Federation 2All-Russian Research Institute of Genetics and Breeding of Farm Animals, St. Petersburg – Pushkin, Tyarlevo, Russian Federation 3Department of Obstetrics and Gynecology, Faculty of Veterinary Medicine, St. Petersburg State Academy of Veterinary Medicine, St. Petersburg, Russia
DOI : https://dx.doi.org/10.13005/bpj/801
Abstract
The placental insufficiency is regarded as the primary factor contributing to late-term abortion and perinatal death of foals. The aim of the present study was to create the predictive model in mares with placental insufficiency. Based on the results of the screening investigation of 136 mares, the treated protocol was created for correction of pathological conditions in the mares with placental insufficiency. We used hematological and biochemical assay to search blood samples, biochemical study of amniotic fluids, clinical examination of electrolyte metabolism, fetus cardiotocography and histological study of postdelivary placental tissues. The link between morphological and physiological changes in placenta, damage of dam`s metabolic proses and fetus distress was proven. The firs sing of the disorders were changes in microcirculation of the placentae. Based on these results was created the treated protocol for correction of placental insufficiently by the use of hypovolemic complex of medicine. As a result of the application of complex intensive treatment of placental insufficiency with ethoxylated starch solution to pregnant mares, it became possible to prolong pregnancy in 88.0% of cases at this study, to get a viable fetus and to prevent perinatal loss. Well-being of pregnancy mainly depends on the ability of placenta to perform their functions. Any changes in the structure of the placenta affect the health of dam`s organism. Evolutionarily, It is justified that in the case of disorders of physiological relationships in fetoplacental complex, internal reserves will be aimed at saving the mother's life. This leads to abortion or premature birth. Placental insufficiency is a severe poliethiological pathology of gestation. Creating a single guideline for the treatment of mares with this pathology is not advisable. The best approach is to create a screening study protocol for identification of the status of a pregnant mare. It will allow supporting a corrective therapy and saving a fetus.
Keywords
Predictive; Pregnancy; Development; Thoroughbred; Mares; Insufficiency; Correction
Download this article as:| Copy the following to cite this article: Avdeenko V. S, Rodin P. V, Volkov A. A, Molshanov A. V, Plemyashov K. V, Potapova A. A Predictive Model for Pregnancy Development in Thoroughbred Mares with Placental Insufficiency and Its Correction. Biomed Pharmacol J 2015;8(2) |
| Copy the following to cite this URL: Avdeenko V. S, Rodin P. V, Volkov A. A, Molshanov A. V, Plemyashov K. V, Potapova A. A Predictive Model for Pregnancy Development in Thoroughbred Mares with Placental Insufficiency and Its Correction. Biomed Pharmacol J 2015;8(2).Available from: http://biomedpharmajournal.org/?p=3185> |
Introduction
The equine placenta is a main component of gestation. The histological background to the study of the equine placenta has been summarized by Steven (1975) and Steven & Morris (1975). The events of biochemical activity of equine placenta have been described by Wooding & Flint (1994). Allen & Stewart (2001) whose laboratory has perhaps contributed the most information to our understanding of this interesting organ.
The equine placenta allows the transfer of nutrients and oxygen from the dam’s blood to the fetus and a diffusion of waste products from the fetus to the dam’s blood. This is the only one way to get the necessary sources for foal, including immunoglobulins. The placenta also synthesizes steroids, peptides, glycoproteins and other biological active molecules, and inactivates other hormones such as catecholamines, glucocorticoids, thyroxine and prostaglandins (Carol, Allen, Steven 1976).
These two functions of transfer and resynthesis of necessary sources for foal`s and dam`s maintains the well-being of gestation. In the case of placenta`s dysfunction, all the physiological relationship between mother and fetus suffers. The full metabolic functioning of placenta does not only provide the normal development of tissues, but also the correct functioning of the entire mother-placenta-fetus system. Metabolic imbalance, occurring in the placenta at the molecular and cellular levels, leads to the development of fetoplacental insufficiency that accompanies almost all pregnancy complications (Potapova et al 2014; Serov 1996).
Placental insufficiency is a serious complication of pregnancy. A review of case records of 3500 aborted fetuses, still-born foals, and foals that died within 24 hours of birth revealed that over 60% of the cases were associated with some form of placental insufficiency such as placentitis, premature placental separation, or placental thickening (Giles et al. 1993). Untreated placentitis can lead to premature delivery, neonatal sepsis and peripartum hypoxia, which leads to either mortality or survival with expensive intensive care. (Ryan 1997)
Another factor that contributes to placental insufficiency is the dysfunction of microcirculatory. The extragenital symptoms of placental insufficiency associated with vascular disorders disappear after the delivery (Avdeenko, 1998; Sugimoto 2003). During vasculogenesis in placental tissues, new capillaries are formed through cell migration and differentiation of endothelial progenitor cells. These processes are controlled by the vascular factors such as vascular endothelial growth factor (VEGF), fibroblast growth factor (bFGF) and its receptor VEGF-R and FGF-R. The changing of balance of cytokines and growth factors, vascular endothelial cells in the microenvironment is the basis of pathological disorders placentation and gestational (Forbes 2010; Maynard 2003; Sugimoto 2003). Many researchers wrote about an important role of imbalance of pro-angiogenic and anti-angiogenic factors in the development of placental insufficiency. The researchers believe that the placenta is the trigger for the occurrence of endothelial cell damage. Insufficient placenta produces a variety of factors that could damage the endothelial cells. Moreover, it leads an imbalance of vascular growth factors and contributes the further increase of microvascularization disorders (Avdeenko 1998, Forbes 2010).
The treatment of pregnant mares with severe placental insufficiency is one of the most difficult problems of veterinary obstetrics. The mechanism of the placental insufficiency development is rather complicated. The concept of chronic hypovolemic shock, accompanied by endotoxemia syndrome and multiple organ dysfunction syndrome, has recently gained quite a strong position, both from theoretical and practical points of view (Kolchin 2009). The methods of pathogenetic therapy of severe placental insufficiency are based on this concept.
According to modern concepts, placental insufficiency should be treated with a new generation of plasma expanders with hemodynamic and rheological properties and minimal side effects (Friedmann 1994). These drugs should be considered as an ideal infusional environment for correction of hypovolemia, hemorheological disorders and colloid osmotic failure in the presence of endothelial damage (Schaap 1996).
The progression of placental insufficiency in pregnant mares is influenced by the level of hypovolemia. To prevent critical levels of hypovolemia a correct infusion and transfusion therapy should be selected (Avdeenko, 1999).
The 6.0% solution of ethoxylated starch is an ideal plasma-solution, it quickly restores the reduced volume of circulating plasma, helps to normalize blood coagulation processes, restores volemic parameters, gives a sufficiently long vascular effect, improves blood rheology, is easily metabolized and excreted from the body and is well tolerated. The effect of the 6.0% haes-steril solution on the clinical course and outcome of pregnancy with severe forms of placental insufficiency has not previously been studied.
The goal of the research was to conduct obstetric clinical examination of pregnant mares in order to study the prevalence of disorders in pregnancy and analyze their causes, as well as to study the efficiency of 6.0% solution of ethoxylated starch (haes-steril produced by “Fresenius”, Germany) in the complex treatment of placental insufficiency in mares.
Materials and methods
The design of the research was chosen in accordance with GLP rules and international recommendations of ICH M3. The research was performed in accordance with the national guide for the care and use of laboratory animals. All manipulations with animals were performed by qualified veterinary doctors. Thus, animal experimentation was approved by the respective local authority.
The study consisted of two parts. The aim of the first part was to conduct the diagnosis of placental dysfunction. The aim of the second part was the treatment of the chosen mares. Outcomes of the treatment were examined by the same methods as in the diagnosis of disorders.
Clinical examination was performed in 136 mares during two years in three breeding stable. The examination was performed as a full study and in order to determine the general condition of the animals. 36 mares in late pregnancy (9 – 11 month of gestation) with placental insufficiency were chosen.
The diagnosis of placental insufficiency was proven by the following methods:
- Blood sampling. Blood samples for hematological studies were taken before the morning feeding from jugular vena. Biochemical blood tests were performed with the analyzer CIBA – CORNING 288 BLOOD GAS SYSTEM (USA). In the analysis of clinical samples, standard methods of veterinary medicine were used.
- Test of amniotic fluid. The amniotic fluid for the study was obtained through transabdominal amniocentesis, which was carried out under the ultrasound control (Sonofine EUS B2, China). The amniotic fluid was transferred directly from the syringe into the chamber of Radelkis Micro-Analyzer 01-215, which made automatic calculation of parameters of acid-base compounds. Subnormal pO2 value of amniotic fluid with normal pCO2 and pH was considered as an indicator of fetal hypoxia of the low level. Reduced pH and pO2 with normal pCO2 was considered as a sign of fetal hypoxia of the medium level. The increase of pO2 to normal or higher levels with low pH showed fetal hypoxia of the high level, the same for high pCO2 with low pH values.
- Control of water and electrolyte balance. It carried out with such factors as the daily output of urine and the presence of edema in the limbs on the mornings.
- Fetal cardiotocography (CTG). We used of fetal Doppler ultrasound for assessment of fetal well-being (Fetal Monitor 155, Corometrics Medical Systems, Inc, Wallingford, Conn, USA). The Fetal Heart Monitor records fetal heart rate and heart rate variability (external Doppler probe), and uterine contractions (external probe with pressure sensor). In the CTG evaluation, the presence of reactive/non-reactive non-stress test was taken into account. Along with the evaluation of the non-stress test, the CTG score was counted. It included acceleration amplitude and instant oscillation amplitude, quality and quantity of decelerations.
- The samples of placenta tissues were collected immediately after the delivery and then fixed in a 10% formalin solution, dehydrated in alcohols in increasing concentration and embedded in paraffin through chloroform. The sections were stained with hematoxylin and eosin. The morphohistometric study of placentae was performed by light and scanning microscopy using the light-optical microscope Cag12е155 (Russia) at magnification rate 200 and 400. A digital camera Axio Scope A 1 was used for microphotography, meanwhile, the average surface of the villi under the microscope was counted. The specific volume of the vascular bed was used as a histological objectively accurate criterion to measure the blood level in the villi.
The second part of study consist of correction of hypovolemia with 6.0% haes-steril – plasma replacement colloidal solution with osmolarity of 308 mOsm/kg, colloid osmotic of 36 mmHg in a dose of 10 mL/kg/d, with concentrated carbohydrate solutions in a dose of 5 mL/kg/d. Fresh frozen plasma was used if the total protein level was less than 60 g/l. Antihypertensive therapy was carried out only after volume expander therapy. 25.0% magnesium sulfate solution was used intravenously as the drug of choice (12 g of dry matter). This infusion therapy program was carried out for 5 days.
The treatment was performed in the test group of animals (n = 18). Other 18 mares formed the intact control group.
The results of therapy were proven on the 5th and 10 day of the study. Besides, for statistical porpoise, the information about pregnancy outcomes and health of newborn foals were also collected.
Statistical analysis of the data was performed using the standard software Microsoft Excel 2000 for Windows. The mean value (M) and significant difference (SD) were determined. The appropriate differences among the means were compared by Student-paired T-test. Differences were considered significant at P<0.05 and P<0.01.
Results and discussion
All dams and their fetus were alive to the end of the study.
According to the results of the clinical examination during pregnancy, placental insufficiency with such characteristic symptoms as edema, hypertension and proteinuria was diagnosed in 26.4% of cases; premature parturition, which occurred due to excessive training of the pregnant mares – in 16.6% of cases; abortions of mainly traumatic nature – in 2.5% of cases; kidney disease as a consequence of nephropathy – in 35.5%; anemia as a result of vitamin deficiency and microelementosis – in 19.0% of mares. These diagnoses were proven in the first step of the study. The statistical data are presented in the figure 1.
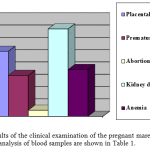 |
Figure 1: Results of the clinical examination of the pregnant mares |
The results of hematological and biochemical assay of mare`s blood demonstrates the imbalance of metabolism of biologically active substances. The decline in the erythrocytes and hemoglobin indicates systemic disorders of hematopoiesis as a result of developing anemia. The value of lymphocytes remains normal. Nevertheless, despite the identification of trends to decline of blood indices, diagnosis for placental insufficiency cannot be confirmed only by the blood tests. Results of the blood tests turn out to be interesting when it is considered in connection with a study of amniotic fluid. Our data indicate that the connection between the metabolism of dams (we learned about it from results of blood samples) and the level of hypoxia of featus, according to the acid-base status of the amniotic fluid (ABS AF), is an established fact. It should be noted that the ABS AF data in their diagnostic efficiency are not inferior to generally accepted methods of fetal monitoring. Thus, the frequency of cases of intrauterine growth retardation and disease incidence of the foals in the neonatal period increases with the growth of fetal hypoxia (Table 2).
Table 1: Parameters of blood samples before treatment
| Parameters | Group | |
| Health mare, (n = 100)M ± SD |
Mare with placental insufficiency, n = 36M ± SD |
|
| Glucose, mmol/l | 4.20 ± 0.31 | 4.17 ± 0.33* |
| Total protein, g/l | 68.58 ± 2.6 | 62.97 ± 2.8* |
| Creatinine, mmol/l | 121.28 ± 8.6 | 116.54 ± 8.1* |
| Blood urea, mmol/l | 5.30 ± 0.5 | 5.44 ± 0.6** |
| Total bilirubin, mkmol/l | 27.8 ± 2.9 | 29.4 ± 2.3** |
| Bilirubin conjugated, mkmol/l | 7.08 ± 0.7 | 7.13 ± 0.8* |
| Serum iron, mkmol/l | 27.48 ± 3.4 | 27.39 ±2.9** |
| Cholesterol mlmol/l | 2.53 ± 0.26 | 2.38 ± 0.13 * |
| Erythrocytes, 1012/l | 8.47 ± 0.34 | 8.52 ± 0.76* |
| Hemoglobin, g / l | 100.25 ± 6.8 | 100.37 ± 6.4* |
| ESR, mm/h | 65.75 ± 6.8 | 65.45 ± 6.5** |
| Leukocytes, 109/l | 8.1 ± 0.6 | 8.0 ± 0.6* |
| Lymphocytes, 109/l | 25.25 ± 0.9 | 24.13 ± 0. 9* |
* – P < 0.05; ** – P> 0.05 – significant differences to the control
Table 2: Values of acid-base status of the amniotic fluid
|
Values of ABS AF |
Health mares
(n=100) M ± SD |
Mares with placental insufficiensy
(n=36) M ± SD |
| рН
рО2 mmHg рСО2 mmHg |
7.14±0.02
97.7±7.41 34.1±1.49 |
7.03±0.01
39.6±2.29 37.5±4.3 |
P < 0.05 – significant differences to the control
The totality of these changes suggests that the main causes of disorders in fetuses during pregnancy are deterioration of nutritional conditions and lack of oxygen supply of the fetus due to metabolic transformations in the mother.
Unfavorable intrauterine growth largely influences the state of the newborn foals. All animals at birth show signs of physiological immaturity characterized by hypotrophy (weight less than 45 kg for Thoroughbred), overall retard in development and resistance weakening (independently does not rise to his feet).
Urine output increased in all the mares suffering from placental insufficiency.
The main prerequisites for the diagnosis of placental insufficiency were the results of cardiotocography with Doppler. There is violation of the bloodstream with a decrease in the signal from the sensor Doppler.
To sum the results of first part up, we can emphasize the main changes in the organism of the mares with placental insufficiency. There is the imbalance of protein and carbohydrate metabolism due to lower eritropoezis. Changes in the dam exist in a relationship with the changes in the body of the fetus. At these physiological circumstances, the placenta is unable to cope with the functional load. Compensatory reactions manifest in the discoordination in the vessels growth in the placenta. This leads to hypoxia and appearing of hyperacidity metabolic products in amnion fluids. This condition more damages dam`s metabolism, which leads to changes in the placentas tissues and the manifestation of clinical signs of placental insufficiency. The most reliable characteristic of placental insufficiency can be the appearance of acidic products in the amniotic fluid and a reduction of placental vascular bed in Doppler assay.
The second part of the study gave the information about the clinical efficacy of the antihypovolemic treated protocol in mares with placental insufficiency.
First of all, an increase in urine output up to 41.7 ± 4.9% (p <0.01) was noted with respect to the initial value before treatment. Swelling disappeared in 89.6% of pregnant mares and decreased in 11.0% (p ≥0.05). Proteinuria decreased from 2.72 ± 0.2 to 0.21 ± 0.1 g / L (p <0.01) or completely disappeared after the treatment.
The parameters of utero-placental blood flow and the nature of cardiac activity served as indicators of the therapy effectiveness in the fetus.
Some abnormality in utero-placental blood flow in pregnant mares was detected prior to the therapy. After the therapy a significant improvement in blood flow in the uterine arteries and umbilical artery was established due to a decrease in peripheral resistance and in diastolic blood flow velocity in response to the improvement of perfusion of the placenta.
The changes in dopplerometric values in the test and control groups are reflected in Figure 2. CTG registered an increase and a decrease in the amplitude of the instant oscillation frequency, a decrease in the duration and severity of spontaneous decelerations, a decrease in the rate stability and an increase in its variability.
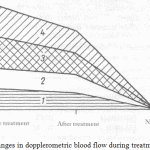 |
Figure 2: Changes in dopplerometric blood flow during treatment |
Fig.2. Changes in dopplerometric blood flow during treatment. Where 1 – SDR (uterine artery, mares up to 8 y.o.), 2 – SDR (uterine artery, mares older than 8 y.o.), 3 – SDR (umbilical artery, mares up to 8 y.o.), 4 – SDR (umbilical artery, mares older than 8 y.o.). On the axis of ordinates the velocity of the flow is indicated in cm/sec.
Furthermore, histological examination of placentae was conducted to evaluate the effect of the treated protocol. Histological examination of placental tissues in the control group revealed some significant compensatory-adaptive reactions: thinning of the interstitial tissue, protein and fatty degeneration of trophoblast cells, vascular obliteration of the villi (Figure 3). Chorionic maturity corresponded to the gestational age (the ratio between vascular-stromal and parenchymal elements). Chorionic villi were shortened, covered with a layer of prismatic epithelium. Compensatory reaction manifested in the presence of necrotic capillaries and fibrinoid deposition where vessels touched the epithelium. A small number of vessels, located in small groups, is also seen. Trophoblast layer is thinned due to reduction of the amount of interstitial tissue. Connective tissue stroma is poor in cellular elements.
The results of the studies of the fetal part of the placenta show the capillary blood flow recovery in mares. The integrity of the epithelial layer, represented by high prismatic cells, is maintained. Such phenomena as necrosis or fibrination of blood vessels are absent. The number of collagen fibers in relation to the cellular elements in the interstitial tissue decreased.
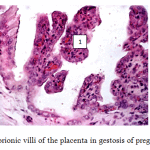 |
Figure 3: Chorionic villi of the placenta in gestosis of pregnant mares |
Fig. 3 – Chorionic villi of the placenta in gestosis of pregnant mares. Stained with hematoxylin and eosin, x200. Brightly colored dilated blood vessels of villi (1) with tissue infiltration of blood cells. Syncytium layer is not visible.
The number of blood vessels and their lumen increased. At the same time, a moderate swelling of the tissue and an infiltration of the interstitial tissue by blood cell elements are present. All the blood vessels are filled with blood; no signs of thrombosis are found (Fig.4).
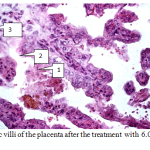 |
Figure 4: Chorionic villi of the placenta after the treatment with 6.0% ethoxylated starch solution. |
Fig.4 – Chorionic villi of the placenta after the treatment with 6.0% ethoxylated starch solution. Stained with hematoxylin and eosin, x200. Clearly visible: syncytium elongated cells (1), basic villi membrane (2), vascularization of chorionic villi (3).
In late gestation, the maximum convergence of the blood vessels of mother and fetus occurs due to the alteration in the endometrium and chorionic epithelium and reduction of villi connective-tissue formations. However, if the structure of the placental tissue is changed, it is not able to mature to a level corresponding to the 300 – 360th day of gestation.
The correction of metabolic processes using 6.0% ethoxylated starch solution is aimed to activate the angiogenesis in tissues of the placenta. The studies have proven the vasoprotective effect of the drug. Basic pathogenetic stages are conditioned by the main causes of placental insufficiency, which are: vascular lesions, impaired synthesis and balance of proteins initially in the placenta and afterwards the generalization of these processes in other vital organs (Collinson and Donnelly 2004).
Thus, in severe forms of placental insufficiency some changes indicating compensatory-adaptive reactions aimed to reduce hypoxia are noticed after the treatment with 6.0% ethoxylated starch solution. As a result of the application of complex intensive treatment of placental insufficiency with ethoxylated starch solution to pregnant mares, it became possible to prolong pregnancy in 88.0% of cases at this study, to get a viable fetus and to prevent perinatal loss.
These studies suggest that acute placental insufficiency may occur in metabolic disorders that damages the microcirculation of the placenta in the presence of generalized vascular lesions. The presence of generalized vascular lesions in mares shows that water-electrolyte and metabolism of biological active substance (amino acids, vitamins) has sequelae of generalized disease because maximum involvement of placental tissue in angiogenesis and local synthesis of potent substances that support the transfer of the sources between the mother and the fetus. (Carol et al 1976; Coignoul and Cheville 1984)
Evidence that concentration of protein and glucose in dam`s blood decreases while total edema and increased diuresis is presented proven the theory of elution of that sources through the kidneys as a result of similar damage to the kidney microvasculature. (Coignoul and Cheville 1984; Strizhova 1996) Further changes in the kidneys and pathological redistribution of body fluids are supported by the nature of the lesions. It provides changes in the morphological structures in the placenta. It is characterized by the decries of numbers of functional chorionic vessel and vascular stromal ratio. If these changes do not accompanied by signs of inflammation, we can talk about non-communicable lesions of the placenta (Avdeenko 1996). The lesions have been attributed to deficiencies in placental blood supply (Coignoul and Cheville 1984, Potapova et al. 2014).We believe that abortion in placental insufficiency may occur as a result of disorders of hemodynamics of the mare. Decreased blood supply to placenta and fetus can result from mechanical compression on blood vessels by edema in myometrium (Coignoul and Cheville 1984).
In this study, lesions of the placental tissues did not appear to correlate with abortion and yielded correct by ethoxylated starch solution. There is the method of treatment of chronic placental insufficiency including infusion therapy (gemoderivat deproteinized blood, hexobendinum + aethamivanum + aethophyllinum) and the use of tocolytics and spazmolitics, sedatives medicines, normalizing blood clotting parameters in the presence of hemostasis disorders (Friedmann 1994).The disadvantage of routine methods of therapy of placental insufficiency is low efficiency expressed in the form of placental insufficiency (with morphological changes in placental tissue) and the individual intolerance of drugs. (Schaap 1996) This disadvantage is avoided in the case of use of 5 sessions of plasmapheresis with partial removal of circulating plasma with solutions of 6.0% ethoxylated starch solution. These is a minimum of side effects, along with their inherent haemodynamic and rheological properties and should be considered at this stage as an ideal medium for infusion correct hypovolaemia, and hemorheological disorders colloid osmotic insufficiency.
Acknowledgment
The authors would like to thank the collaborators from Kudashevsky stud farm Ltd. and Petrovsky State Stud Farm for collecting blood and placentae samples from mares. We give our special thanks to Biochemical Laboratory of Saratov State Agricultural University for substantial help in biochemical and histological assays. We also acknowledge the language editor Dr Daria Serés for significant revision of the manuscript.
References
- Allen, W.R. and Stewart, F. (2001) Equine Placentation. Reproduction, Fertility and Development, 13, 623-634.
- Avdeenko, V. Antenatal diagnosis of fetal hypoxia as a result of the study of amniotic fluid / Actual problems of veterinary surgery. Abstract. dedicated to the 70th anniversary of CAF. surgery, VSAU them. KD Glinka //.- Voronezh.- 1999.- S.5-6.
- Avdeenko V, Diagnosis of placental insufficiency in pregnant mares with extragenital pathology / Proceedings of the Intern. scientific. Conf., is dedicated. 125th anniversary of the Academy of //. – Kazan, 1998.- S.108-109.
- Carol, A.S., Allen W.R., Steven D.H. Studies on the equine placenta. II. Utrastructure of the placental barrier // J. Reproduction Fertility. 1976. Na 48. P. 257 – 264.
- Coignoul, F.L., Cheville N.F. Pathology of the maternal genital tract, placenta, and fetus in equine viral arteritis // Veterynary Patology Online. 1984. N? 21. P. 333 – 340.
- Collinson D., Donnelly R. Therapeutic angiogenesis in peripheral arterial disease: can biotechnology produce an effective collateral circulation? Eur. J. Vasc. Endovasc. Surg. 2004; 28: 9-23.
- Forbes K., Westwood M. Maternal growth factor regulation of placental development and fetal growth. Journal of Endocrinology, 2010; 207: 1-16.
- Glade, M. Dietary yeast culture supplementation of mares during late gestation and early lactation. 2. Effects on milk production, milk composition, weight gain and linear growth of nursling foals / M. Glade // Journal of Equine Veterinary Science. ― 1991. ― V. 11. ― N. 2. ― Р. 89‒95.
- Friedmann G., Jankowski S., Marecaux G. e.a. Hemodynamic effects of two different hydroxyefhylsrach solutions (6%, 10%) in septic patients. Intensive Care 20, Suppl 2 (1994).
- Kolchin A Perinatal pathology in animals. – Monograph. – Ekaterenburg. – 2009. – 198 p.
- Maynard S.E., Min J.Y., Merchan J. et al. Excess placental soluble fms-like tyrosine kinase 1 (sFltl) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Invest. 2003; 111: 649-658.
- Potapova A., Muzhikyan A, Bazhenov N, Plemyashov K. Morphological assessment of maturity of equine plasenta in comparative aspects. Questions of regulatory veterinary medicine. 2014, №4. P. 92-95
- Ryan, P.L, Vaala, Wendy and Bagnell. Equine relaxin: a diagnostic tool for placental insufficiency and parturient complication in the mare. Proceedings 15th Equine Nutrition and Physiology Symposium, Fort Wor1h, Texas, 1997, May: 28-31.
- Schaap А.Н. et at. Fetal distress due to placental insufficiency at 26 through 31 weeks: a comparison between an active and a more conservative management. Eur. Obstet Gynecol. Reprod, Biol. 1996, Dec. 70 (I) : 61-8.
- Serov, V., Dobronetskaya D, Ilienko L, Nenakhov F // Problems of gestosis: Abstracts. – Cheboksary, 1996. – S. 86 – 89.
- Steven DH and Morris G Development of the foetal membranes. In: Steven DH (ed) Comparative Placentation, New York: Academic Press, 1975, pp.214-67.
- Strizhova, N, Duguay A, Fomin M. et al. // Problems of gestosis: Abstracts. – Cheboksary, 1996. – S. 180 – 186.
- Sugimoto H., Hamano Y., Charytan D., Cosgrove D., Kieran M., Sudhakar A., Kalluri R. Neutralization of circulating vascular endothelial growth factor (VEGF) by anti-VEGF antibodies and soluble VEGF receptor 1 (sFlt-1) induces proteinuria. J. Biol. Chem. 2003 Apr 11; 278 (15): 12605-8.
- Wooding FBP, Flint APF: Placentation. In Marshall’s Physiology of Reproduction, Volume 3. 4th edition. Edited by Lamming GE. London: Chapman and Hall; 1994:233–460.







