Elham Sadeghi
Department of Surgery, Medicine Faculty, Islamic Azad University Tehran Medical Branch, Tehran, Iran
DOI : https://dx.doi.org/10.13005/bpj/603
Abstract
Breast cancer is the most common cancer in women and the leading cause of cancer death in women with 40-44 years old.The facts and evidence show steadily increasing incidence of breast cancer in the mid-1940s.That's why we conducted this study to evaluate the survival rate of patients,positive estrogen receptor in breast cancer patients treated over the last 10 years in Shohada Hospital in Tajrish, Tehran have been selected as a case study.In this study,the data were collected using a descriptive– analytical method andusing the SPSS software, we came to the conclusion that there is no significant relationship between survival and condition of patients after treatment of patients with menopausal status as well as the type of treatment with the patients' survival rate.If a significant relationship has been observed between the patients after treatment, it means that the disease has been diagnosed with stage.The results ofANOVA test also show that there is a significant correlation between the diagnosis of observed disease age and survival of patients.
Keywords
breast cancer; tamoxifène; estrogen; National Cancer Institute; Shohada Hospital of Tajrish
Download this article as:| Copy the following to cite this article: Sadeghi E. Study Of Positive Estrogen Receptor Breast Cancer Survival Of Patients Treated At The Shohadahospital In Tajrish, Tehran. Biomed Pharmacol J 2015;8(1) |
| Copy the following to cite this URL: Sadeghi E. Study Of Positive Estrogen Receptor Breast Cancer Survival Of Patients Treated At The Shohadahospital In Tajrish, Tehran. Biomed Pharmacol J 2015;8(1). Available from: http://biomedpharmajournal.org/?p=792 |
Introduction
America Cancer Society estimates that each year of 180/ 000 women in the United States are affected with breast cancer and die from the disease for more than 40/000. For many years, researchers are studying the best way to find breast cancer therapy, and special attention has been paid to the prevention of recurrence of the disease after initial treatment.Hormone therapy is a method that cancer cells are deprived from estrogen that some breast cancer cells need to grow.Based on the National Cancer Institute model, a 5 years of preventive treatment with the drug tamoxifènecitrate, as adjuvant hormonal therapy reduces the risk of invasive breast cancer up to a high percentage and significantly. Tamoxifène was first built as a project for the creation of oral contraceptives. But due to anti-estrogen actions, it has become a candidate for endocrine treatment of breast cancer.The success of treatment with tamoxifène in reducing the incidence of breast cancer in patients with contra lateral breast cancer led researchers to further explore the use of taking tamoxifènet o prevent cancer. Studies show that chemotherapy plus the use oftamoxifène resulted in disease free survival compared to the more obviously the use of tamoxifène alone. Although,tamoxifène is a suitable drug as an anti-estrogen in breast tissue, but is acts as the estrogen on bone properties,cardiovascular system, endometrial, ovarian and possibly liver and benign lesions of the uterus,endometrial lesions, endometrial cancer and ovarian cancer are more common side effects of the drug early.
Research Objectives
- Review of correlation between age and lifetime of the patient’s initial treatment with tamoxifènein patients with breast cancer
- Study of the difference in the outcome of treatment with tamoxifènein postmenopausal women and non-menopausal breast cancer patients and lifetime of patient
- Examining the results of the selected therapy to the patient’s age and the patient’s lifetime in patients with breast cancer
- Investigating the effect of treatment with tamoxifènein breast cancer and its impact on women and neoplastic disease of the patient’s lifetime in patients with breast cancer
Methodology
This research is descriptive – correlational analysis (meta-cognitive).
Statistical population
The research population consisted of all patients with positive estrogen receptor breast cancer that have been under various treatment methods in the last 10 years at Shohada hospital in Tajrish, Tehran,thus, data collection was carried out through the whole number (250 patients).
Tools and methods for data collection
demographic data and disease records were extracted from the medical records and analyzed using SPSS statistical software.
Data analysis
analysis of parametric and non parametric data was performed through statistical analysis.
Literature review
Breast cancer and its risk factors
Breast cancer is the most common cancer in women and the leading cause of cancer death in women with 40-44 years old. These malignancies makes up 33% of women with cancers and is responsible for 19% of deaths related to cancer. Facts and evidence show steadily increasing incidence of breast cancer in the mid-1940s. Diagnosis of breast cancer is one of the most unpleasant events may occur during a woman’s life. According to doctors,the fear of breast cancer epidemic is a normal reaction and is perhaps a part to raise awareness about the disease returns. While the patients, except for the physical, areal so grappling with the psychological effects of breast cancer.In this disease, malignant proliferation of epithelial cells lining the ducts or lobules of the breast will occur.Like all epithelial malignancies, the incidence of breast cancer increase with age gradually,but the age of menstruation slope of the curve decreases. Age at menarche,first pregnancy and menopause, three important dates influence on the occurrence of breast cancer in women.The lower age at menarche, age at first pregnancy and the higher age of menopause,the risk increases that these cases represent the sex hormone dependence of cancer.Women whose age at menarche was 16 years old, about 50-60% of people at age 12 have been diagnosed with breast cancer.Similarly, the risk of cessation of menstruation in women over the age of 10 years earlier than the average of its natural age (52 years), reaches to 35% of others by surgery alone, or in women who have oophorectomy,early menstruation is a risk of breast cancer that is one in three cases that nature menopause occurs at age 50 or older.The first two periods in older age and lower gestational age,are important protective factors against breast cancer in older ages.Thus, the presence of these two factors, the incidence of this cancer is oriented towards a younger age.In the meantime, especially since the first full-term pregnancy, safety is the most important factor.Based on the research results, the risk of breast cancer reduced at an early age than non-pregnant women conversely, increasing the risk later in life.Geographical differences in the incidence and mortality from breast cancer have shown that risk factors for the disease varies in different areas for breast cancer, mainly environmental factors are stronger than genetic factors.
The most important factors in breast cancer are those patients with staging.On the other variables such as estrogen and progesterone receptors can be noted.Tumors lacking this receptor are more likely to have a recurrence. Criteria for tumor growth,classification of tumors according to histological, molecular changes in tumors proteins are involved in the invasion of the rest of influential factors.In the case of cell therapy selection including chemotherapy and hormone therapy remains controversial.For different nodes of patients, different therapeutic regimens have been set.
Treatment Methods
- Initial treatment
Initial treatment of breast cancer involves removing the lumpectomy, radiation therapy, modified radical mastectomy. Lumpectomyoperation is to remove the tumor mass and low levels of the tissue surrounding the breast. Usually, most of the underarm lymph nodes are also removed. Following the lumpectomy, breast radiation therapy is done.
- Modified radical mastectomy
involves removing the entire breast and axillary lymph nodes, most often covering the chest muscles. A new technique called sentinel lymph node biopsy is a biopsy, in which only one of the lymph nodes removed and the spread of breast cancer cells is studied. Clinical studies show the increasing importance of this method in the treatment of breast cancer.
- Adjuvant treatment
is a supplemental measure that is performed in addition to the initial treatment to patients and aims to destroy the cells spread however, laboratory and radiological procedures may not be able to detect these cells.Adjuvant treatment of breast cancer involves chemotherapy or hormone therapy(Either alone or a combination of both).In most cases, adjuvant hormonal therapy is done with the drug tamoxifène.
Factors affecting disease
Important prognostic factors are indicator of breast masses that can help to predict the likelihood of the disease recovery,some of the factors that are commonly used in breast cancer treatment planning are as follows:
Size of the tumor: In general, patients with small tumors (diameter of 2 cm or less) have a better prognosis than patients with larger tumors (especially diameter of 5 cm).
Lymph node involvement: if breast cancer is a positive lymph node, the risk of recurrence in breast cancer of negative lymph node type is more.
Hormonal receptor status:Studies have shown that approximately 2/3 of all cases of breast cancer contains considerable amounts of estrogen receptors, called receptor, and this is why this masses called positive estrogen receptor (+ estrogen receptor positive = ER). About 40 to 50 percent of all breast cancer cases are progesterone receptors and that is why they “are called positive progesterone receptor. Masses of ER +have less invasive development than the masses ER–.Consequently, the prognosis ofER+ patients is better than ER-.
Histological grading: cancer cells contain very similar to cell natural structure of the so-called well-differentiated breast cancer against that the little resemblance to normal breast cells and the cells are “poorly differentiated” called and cancers in borderline of the two previous cases called”moderately differentiated”.
Activity of cancer-causing gene (Oncogene): the activity of this gene led to the abnormal cells or changing the minto normal cancerous cells. Cancer patients containing an oncogene called HER -2 / neu are at greater risk of recurrence.
Tamoxifen
The most common drug used in hormone therapy,is tamoxifen that is used since 20 years ago. Tamoxifen prevents the combination of estrogen to breast cancer cells, and it is effective to reduce the breast cancer recurrence to other parts of the body. Also this drug has been confirmed in order to prevent breast cancer in women who have a high risk of HIV infection and reducing the recurrence of “ductal carcinoma cancer in place”.
Tamoxifen also affect other cells in the body than cancer cells.Some of the side effects of tamoxifen may occur in rare cases,increased risk of blood clots in the veins of the body, which may move up the clot caused respiratory problems for the patient.Another side effect of tamoxifenis the increased risk of uterine cancer.
Tamoxifen, in addition to the anti-cancerous effects that is the main benefit of this drug, results in reduced blood cholesterol and reduced risk of osteoporosis, which is also among the benefits of this medicine.
Clinical applications of tamoxifen
Tamoxifen,for the first time in 1962, was made as an initiative to make oral contraceptives. Tamoxifen, in 1997, was confirmed for the treatment of metastatic breast cancer, and subsequently, as adjuvant therapy for the disease. The harmful effects profile was well-defined and data of secondary benefits have been collected from 10 million patients in the year. Tamoxifen has become the most widely prescribed and studied as anti-cancer drug in the world (data on file, Zenka Pharmaceuticals, Wilmington, Del., 1999)
Studies showed that treatment with tamoxifen may be helpful for women at all stages of breast cancer,regardless of the status of the node or menopause. Today, the hormone receptor status of the tumor is generally more important factor in treatment with tamoxifen regarding the age, menopausal status, or staging of cancer patients. Other treatment decisions relyon node status, tumor size, presence or absence of metastasis and prognosis of patients.
Tamoxifen prevents both invasive and non-invasive disease and to reduce the incidence of invasive disease in women confirmed with carcinoma in situ. Long-term safety and secondary effects, play an important role in determining which women are likely to benefit the preventive treatment. It is shown that tamoxifen increases bone density and improves lipid profiles, but is linked with the increased incidence of endometrial cancer thromboembolism.
Role of tamoxifen in treating breast cancer
Breast cancer before going to the advanced mode, tamoxifen is often used as adjuvant therapy after local therapy. Clinical studies and meta-analysis, have shown the benefits of tamoxifen in the adjuvant treatment, and a reduction in breast cancer recurrence by 42% and 22% reduction in mortality 10 years after treating the receiving women, have reported 5 years of tamoxifen.
In patients with estrogen receptor-positive tumors treated with tamoxifen for 5 years reduced the risk of relapse by 50% (4% ± SE) and the risk of death by 28% (5% ± SE). Separate clinical studies have shown the benefits of tamoxifen treatment for both patients before menopause node-positive or node-negative disease after menopause are obvious, in addition, in the largest study of women with node-negative disease, estrogen receptor positive, the overall survival benefit of tamoxifen was found after 10 years. (Risk of death, 0/84 to 0/04 = p).
Role of tamoxifen in preventing breast cancer
14-B NSABP study provides evidence that tamoxifen group compared with the untreated controls showed a substantial reduction in the incidence of breast cancer.It seemed that tamoxifen treated breast cancer at such an early stage that do not clinically prevent the formation of tumors seriously.In the United States, according to 1-p NSABP study and consideration of other information,tamoxifen have been confirmed to reduce the risk of breast cancer women who gain the risk of the disease.
Breast Cancer Prevention
The risk of breast cancer by modified method of the National Cancer Institute described by Gill and Benichu used in the NSABP study. In general, the higher the risk of breast cancer, the benefit of treatment is prevention. Experts at the National Cancer Institute, breast cancer cases are preventable 1-p NSABP study on the risk of invasive breast cancer in the next 5 years, 97 cases of breast cancer per 10,000 women taking tamoxifen each period of the receiver were preventable.When the risk to 4/0% increase over the 5-year period, only 52 women were needed to treat to prevent one case of breast cancer, and when the risk is 7/0 percent, 1 case of breast cancer was treated for every 30 women received tamoxifen at the same level of risk. If prevention Non-invasive form of breast cancer were analyzed, the results were even the most effective. The relative benefit of tamoxifen in women who received HRT and breast cancer in women with specific genotypes is not yet known.For all women at increased risk of breast cancer, the benefits of prophylaxis with tamoxifen should be weighed against the potential risks. Women with atypical hyperplasia, LCIS or DCIS may be candidates for preventive treatment. Women younger than 50 years may have reduced the increased risk of breast cancer receiving treatment before menopause choose to risks of thromboembolism following menopause increases the risk of endometrial cancer. Women older than 50 years by embolism or thrombosis risk now are probably good candidates for preventive treatment. Women are at an increased risk for preventable treatment. Other women at increased risk for breast cancer should be considered for prophylaxis period, if the benefits are preferable to the risks.
Literature review
Study of 14-B national design of adjuvant Breast and Bowel Surgery (NSABP) to examine the effects of tamoxifen treatment over 5 years to 10 years concluded that there is no significant difference between 5 and 10 years of disease free survival and overall survival. In other results that benefit of 5 years of tamoxifen treatment, with 10 years’ worth pursuing.
A meta-analysis conducted by the working group trialist primary breast cancer, data from a randomized study of adjuvant tamoxifen consumption were examined prior to 1990. The latest results were published in 1998. The reduction in breast cancer recurrence and mortality was more for women who received 5 years of treatment than those who received 1 or 2 years of treatment. A meta-analysis showed that 5 years of treatment, the incidence of endometrial cancer increased. This is a 42% reduction in the incidence of breast cancer in women treated disagree for 5 years tamoxifen opposed balance.
In Italian tamoxifen prevention study, women were selected who had a previous hysterectomy randomly to receive tamoxifen citrate 20 mg daily or placebo. For 5 years, with 5 years of follow-up planned, can be selected. The study was stopped because of high levels of exclusion, with only 149 (3%) of a person who has a 5-year analysis of 54,090 women completed the treatment and 3,837 (71%) participants who has received intervention. The average follow up of 46 months shows that there is no difference in breast cancer incidence between the placebo and tamoxifen groups. Hormone replacement therapy (HRT) used by 14% of women during the study. The women, statistically significant reduction (0/0216 = p) in the incidence of breast cancer was not found the total population.
A pilot study that was conducted at the Royal Hospital in London Marsden compared the incidence of breast cancer in healthy women between the ages of 30 and 70 years of age who received tamoxifen or placebo. Eligibility was based on having a family history of cancer. Participants study received tamoxifen citrate 20 mg daily or placebo for 8 year.
After an average 70 months of follow-up, the overall incidence of breast cancer for patients receiving tamoxifen was similar as medicine recipients. Unlike the Italian study, the study showed no effect of the receptor for tamoxifen in women taking HRT. 12 of 523 breast cancer set up in patients of tamoxifen group who received HRT and placebo groups in 13 of the 507 individual code HRT receiving (0/6= p). Five cases of endometrial cancer were reported.
Differences between the results of NSABP p-1 Great Britain are more complicated, because the study of women with at least a few risk factors for breast cancer were enrolled. Royal Marsden researchers estimated an increased reduction about 50%, 90% respectively. NSABP Royal Marsden study authors noted that the population may be susceptible to estrogen receptor-negative tumors in the population are tested by the NSABP. Since the effects of tamoxifen depends on estrogen receptor positive status, and because the study was based on the assumption prevent the population difference might leading to a significant change in the overall strength of the study. Another significant difference between the 2 studies, use of HRT by 26% of participants of Great Britain compared with no use of HRT in the NSABP study participants. Since tamoxifen natural estrogen receptor on the tumor compete, concurrent endocrine treatment can interfere in the results.
Finally, the Royal Marsden study, enrolled women based on family history, participants were more likely to have a genetic component in the risk factors.
Information from the NSABP study showed that the patients with DCIS who had a lumpectomy, and 25% over the next 5 years with mammary tumors, while only 13% of those who underwent a lumpectomy and radiation therapy, during which were affected by breast cancer respectively.
24-B NSABP study found that the addition of tamoxifen to lumpectomy and radiation diet, decreased breast cancer rates of 5-year-old all first events of 13/4% (placebo) to 8/2% (0/001<p). In the tamoxifen group, only 4/1 percent of DCIS patients were affected by invasive cancer after 5 years, slightly was more than half (2/1% of the total) in the same breast.
According to a study conducted at Harvard University in 2002, the 5-year study of the effect of tamoxifen in postmenopausal women with 50 years old, women without a uterus received a good life expectancy. If postmenopausal women with a uterus, such an achievement if they had breast cancer (1).
According to a study that was conducted in 2007 in England, it was announced that an aromatase inhibitor to tamoxifen or rather was given to the women with ER +shows early progress in saving the lives of patients (2).
According to a study that was conducted in 1998 in England, it was announced that tamoxifen reduces breast cancer recurrence in women with ER + and the increase in survival at 10 years old but information is incomplete about the ER– (3).
According to a study in 2002 at Columbia University on 4 groups of women with atypical hyperplasia, it was announced that chemotherapy and tamoxifen can be especially useful in women with Atypic hyperplasia. As well as the use of tamoxifen has been more useful in women under 50 years and then (4).
According to a study that was conducted in 2000 in America, it was announced that treatment with tamoxifen increases the risk of endometrial cancer and thromboembolism (5).
According to a study that was conducted in 1995 in America, it was announced that the use of tamoxifen in a short time is not associated with an increased risk of uterine cancer, but is associated with a reduced risk of breast side (7).
According to a study that was conducted in 1996 in Texas, it was announced that tamoxifen acts as an anti-estrogen in breast tissue, but estrogenic properties on bone, cardiovascular system and liver, and the women 2 to 3 times the general population are at risk for endometrial cancer (8).
According to a study that was conducted in 1995 at the University of Washington, it was said that the incidence of side effects of tamoxifen depends on the duration of treatment (9).
According to a study that was conducted in 2004 in Texas, it was announced that patients with both types of receptor HER2 / Neu and Coactivator ALB1 are more resistant to tamoxifen. Research on drug Gefitinib as neutralizing the impact resistance Coreceptor is underway (10).
Analysis
In this section, we will assess demographic information obtained from the patients using SPSS software and ANOVA and Chi Square test.
Statistic description of data and information:
Age: The average age at diagnosis stage was 52 years (+ 13/5) and the minimum and maximum age of diagnosis was 25 and 89 years old.
Menopause: female patients with breast cancer, 45/6% were postmenopausal and 54/4% were not postmenopausal.
P53: P53 in breast cancer patients in the 74/4% was negative and 25/6% negative.
HER2: HER 2 receptors in the 42/8% of patients was positive and in 57/2 of patients was negative.
stage of the cancer: In this study, the frequency in patients with IIastage was 26/4%, IIb phase was 18/4%, IIIa and IIIc stages were 14%, phase I 9/6%, stage IV 10/8% and IIIb stage were6 /8% respectively.
Mastectomy: 22/2 have been mastectomy% and 77/8% have not been mastectomy.
Lumpectomy: In this study of 250 patients with breast cancer, 80% have not been lumpectomy and 20% have been lumpectomy.
HRT receptor: in patients with breast cancer, HRT receptor have been reported positive in at 100%.of patients.
Chemotherapy, according to a survey conducted in this study, 31/6% (79 patients) for the treatment have received chemotherapy and 68/4% of patients have not received chemotherapy.
Radiotherapy: In this study, 87/6% (219) were used for the treatment of radiotherapy and 12/4% of patients were not used in the radiation.
Survival of patients: The mean survival time of patients after treatment with a standard deviation of +2.4 was at least 4 years and maximum survival rate of 1 and 12 years. In 6/77% of patients survival rate 1-5 years and 18/8 years and 6-10 survival rate at 3/5% over 11 years.
Status of patients after treatment: in 250 patients with breast cancer after treatment, 92/4% without metastasis and other diseases have been 3/6%,1/2%, recurrence of disease (after two years), 0/8% (2 patients) were affected by ovarian cancer, 1/2% (3 patients) and bone metastases 0/8% (2 patients) had a recurrence in the liver.
Table 1: Descriptives- Age of diagnose
|
|
N | Mean | Std. Deviation | Std. Error | 95% Confidence Interval for Mean | Minimum | Maximum | |
| Lower Bound | Upper Bound | |||||||
| 1-5y | 194 | 52.26 | 13.614 | .977 | 50.33 | 54.19 | 25 | 85 |
| 6-10y | 47 | 52.45 | 14.476 | 2.112 | 48.20 | 56.70 | 32 | 89 |
| >11y | 9 | 53.00 | 5.268 | 1.756 | 48.95 | 57.05 | 49 | 60 |
| Total | 250 | 52.32 | 13.538 | .856 | 50.63 | 54.01 | 25 | 89 |
Discussion and review of the data
In this section, the results of tests carried out on information and statistics are provided:
According to ANOVA analysis, a significant association have been observed between age at diagnosis and survival rates. (-0/985 = P)
Table 2: ANOVA – Age of diagnose
| Sum of Squares | df | Mean Square | F | Sig. | |
| Between Groups | 5.670 | 2 | 2.835 | .015 | .985 |
| Within Groups | 45630.730 | 247 | 184.740 | ||
| Total | 45636.400 | 249 |
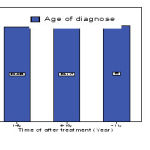 |
Figure 1 |
According to test Chi Square test, there was no significant correlation between survivals of patients with menopausal status of patients. (0/52 = P)
Table 3: of Distribution of patients’ survival rate after treatment on menopausal status of the patients after treatment
| Time of after treatment (Year) * Menopause Crosstabulation | Menopause | Total | |||
| Yes | No | ||||
| Time of after treatment (Year) | 1-5y | Count | 92 | 102 | 194 |
| % within Time of after treatment (Year) | 47.4% | 52.6% | 100.0% | ||
| % within Menopause | 80.7% | 75.0% | 77.6% | ||
| % of Total | 36.8% | 40.8% | 77.6% | ||
| 6-10y | Count | 19 | 28 | 47 | |
| % within Time of after treatment (Year) | 40.4% | 59.6% | 100.0% | ||
| % within Menopause | 16.7% | 20.6% | 18.8% | ||
| % of Total | 7.6% | 11.2% | 18.8% | ||
| >11y | Count | 3 | 6 | 9 | |
| % within Time of after treatment (Year) | 33.3% | 66.7% | 100.0% | ||
| % within Menopause | 2.6% | 4.4% | 3.6% | ||
| % of Total | 1.2% | 2.4% | 3.6% | ||
| Total | Count | 114 | 136 | 250 | |
| % within Time of after treatment (Year) | 45.6% | 54.4% | 100.0% | ||
| % within Menopause | 100.0% | 100.0% | 100.0% | ||
| % of Total | 45.6% | 54.4% | 100.0% | ||
Table 4:Chi-Square Tests
| Value | df | Asymp. Sig. (2-sided) | |
| Pearson Chi-Square | 1.313(a) | 2 | .519 |
| Likelihood Ratio | 1.331 | 2 | .514 |
| Linear-by-Linear Association | 1.308 | 1 | .253 |
| N of Valid Cases | 250 |
a 2 cells (33.3%) have expected count less than 5. The minimum expected count is 4.10.
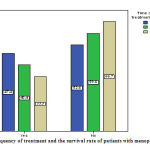 |
Figure 2: Chart of frequency of treatment and the survival rate of patients with menopausal status |
survival rate of 1-5 years in patients with postmenopausal 47.4, and in premenopausal women 52/6%, 6-10years survival rate in postmenopausal women 40/4% , and in premenopausal women 59/6 survival rate was more than 11 years in postmenopausal women 33/3% in premenopausal women was66/7 percent.
Table 5: Status after treatment * Menopause Crosstabulation
| Menopause | Total | ||||
| Yes | No | ||||
| Status after treatment
|
Treated
|
Count | 108 | 123 | 231 |
| % within Status after treatment | 46.8% | 53.2% | 100.0% | ||
| % within Menopause | 94.7% | 90.4% | 92.4% | ||
| % of Total | 43.2% | 49.2% | 92.4% | ||
| Died
|
Count | 3 | 6 | 9 | |
| % within Status after treatment | 33.3% | 66.7% | 100.0% | ||
| % within Menopause | 2.6% | 4.4% | 3.6% | ||
| % of Total | 1.2% | 2.4% | 3.6% | ||
| Relaps
|
Count | 0 | 3 | 3 | |
| % within Status after treatment | .0% | 100.0% | 100.0% | ||
| % within Menopause | .0% | 2.2% | 1.2% | ||
| % of Total | .0% | 1.2% | 1.2% | ||
| Ovarian cancer
|
Count | 0 | 2 | 2 | |
| % within Status after treatment | .0% | 100.0% | 100.0% | ||
| % within Menopause | .0% | 1.5% | .8% | ||
| % of Total | .0% | .8% | .8% | ||
| Bone metastases
|
Count | 3 | 0 | 3 | |
| % within Status after treatment | 100.0% | .0% | 100.0% | ||
| % within Menopause | 2.6% | .0% | 1.2% | ||
| % of Total | 1.2% | .0% | 1.2% | ||
| Liver relaps
|
Count | 0 | 2 | 2 | |
| % within Status after treatment | .0% | 100.0% | 100.0% | ||
| % within Menopause | .0% | 1.5% | .8% | ||
| % of Total | .0% | .8% | .8% | ||
| Total
|
Count | 114 | 136 | 250 | |
| % within Status after treatment | 45.6% | 54.4% | 100.0% | ||
| % within Menopause | 100.0% | 100.0% | 100.0% | ||
| % of Total | 45.6% | 54.4% | 100.0% | ||
Table 6: Chi-Square Tests
| Value | Df | Asymp. Sig. (2-sided) | |
| Pearson Chi-Square | 10.116(a) | 5 | .072 |
| Likelihood Ratio | 13.919 | 5 | .016 |
| Linear-by-Linear Association | .658 | 1 | .417 |
| N of Valid Cases | 250 |
a 10 cells (83.3%) have expected count less than 5. The minimum expected count is .91.
According to Chi-square test, there was no significant association between patients after treatment with menopausal status of patients. (-0/072 = P)
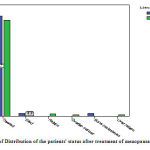 |
Figure 3: Chart of Distribution of the patients’ status after treatment of menopausal status |
inpostmenopausal patients 94.7 treated and in premenopausal women 90.4% were treated, 2.6 menopausal patients and 4/4% ofpremenopausal patients, had died after treatment in 2/2 of recurrence in postmenopausal women 1/5% of premenopausal ovarian cancer, 2/6% of postmenopausal bone metastases, 5/1% were postmenopausal patients who had recurrence in the liver.
Table 7: Status after treatment * Stage Crosstabulation
| Stage | Total | |||||||||
| IIa | IIb | IIIa | IIIc | I | IV | IIIb | ||||
| Status after treatment | Treated | Count | 61 | 46 | 33 | 29 | 24 | 21 | 17 | 231 |
| % within Status after treatment | 26.4% | 19.9% | 14.3% | 12.6% | 10.4% | 9.1% | 7.4% | 100.0% | ||
| % of Total | 24.4% | 18.4% | 13.2% | 11.6% | 9.6% | 8.4% | 6.8% | 92.4% | ||
| Died | Count | 0 | 0 | 0 | 3 | 0 | 6 | 0 | 9 | |
| % within Status after treatment | .0% | .0% | .0% | 33.3% | .0% | 66.7% | .0% | 100.0% | ||
| % of Total | .0% | .0% | .0% | 1.2% | .0% | 2.4% | .0% | 3.6% | ||
| Relaps | Count | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | |
| % within Status after treatment | 100.0% | .0% | .0% | .0% | .0% | .0% | .0% | 100.0% | ||
| % of Total | 1.2% | .0% | .0% | .0% | .0% | .0% | .0% | 1.2% | ||
| Ovarian cancer | Count | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | |
| % within Status after treatment | 100.0% | .0% | .0% | .0% | .0% | .0% | .0% | 100.0% | ||
| % of Total | .8% | .0% | .0% | .0% | .0% | .0% | .0% | .8% | ||
| Bone metastases | Count | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 3 | |
| % within Status after treatment | .0% | .0% | .0% | 100.0% | .0% | .0% | .0% | 100.0% | ||
| % of Total | .0% | .0% | .0% | 1.2% | .0% | .0% | .0% | 1.2% | ||
| Liver relaps | Count | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 2 | |
| % within Status after treatment | .0% | .0% | 100.0% | .0% | .0% | .0% | .0% | 100.0% | ||
| % of Total | .0% | .0% | .8% | .0% | .0% | .0% | .0% | .8% | ||
| Total | Count | 66 | 46 | 35 | 35 | 24 | 27 | 17 | 250 | |
| % within Status after treatment | 26.4% | 18.4% | 14.0% | 14.0% | 9.6% | 10.8% | 6.8% | 100.0% | ||
| % of Total | 26.4% | 18.4% | 14.0% | 14.0% | 9.6% | 10.8% | 6.8% | 100.0% | ||
Table 8: Chi-Square Tests
| Value | df | Asymp. Sig. (2-sided) | |
| Pearson Chi-Square | 81.361(a) | 30 | .000 |
| Likelihood Ratio | 61.587 | 30 | .001 |
| Linear-by-Linear Association | .000 | 1 | .990 |
| N of Valid Cases | 250 |
a 35 cells (83.3%) have expected count less than 5. The minimum expected count is .14.
According to Chi-square test, a significant associationhas been diagnosed between patients after treatment with the disease. (-0.00 = P)
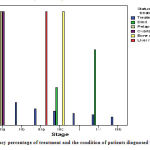 |
Figure 4: Chart of frequency percentage of treatment and the condition of patients diagnosed with the disease
|
The most frequency of treated patients and patients with recurrent ovarian cancer, patients with stage IIa, patients with liver cancer and metastases in IIIastage and IV stage patients were more dead.
Table 9: of relative and absolute frequency of patients with breast cancer
| Type of treatment | Frequency | Percent | Valid Percent | Cumulative Percent | |
| Valid
|
Chemotherapy& Radoitherapy | 140 | 56.0 | 56.0 | 56.0 |
| Chemotherapy | 32 | 12.8 | 12.8 | 68.8 | |
| Radiotherapy | 78 | 31.2 | 31.2 | 100.0 | |
| Total | 250 | 100.0 | 100.0 | ||
Chemotherapy and radiotherapy were usedin 56% of patients at the same time and 12/8% just by chemotherapy and31/ 2% were treated by radiotherapy.
Table 10: of frequency of treatment on survival in patients with breast cancer
| Time of after treatment (Year) * Type of treatment Crosstabulation | Type of treatment | Total | ||||
| Chemotherapy& Radoitherapy | Chemotherapy | Radiotherapy | ||||
| Time of after treatment (Year) | 1-5y | Count | 110 | 27 | 57 | 194 |
| % within Time of after treatment (Year) | 56.7% | 13.9% | 29.4% | 100.0% | ||
| % within Type of treatment | 78.6% | 84.4% | 73.1% | 77.6% | ||
| % of Total | 44.0% | 10.8% | 22.8% | 77.6% | ||
| 6-10y | Count | 24 | 5 | 18 | 47 | |
| % within Time of after treatment (Year) | 51.1% | 10.6% | 38.3% | 100.0% | ||
| % within Type of treatment | 17.1% | 15.6% | 23.1% | 18.8% | ||
| % of Total | 9.6% | 2.0% | 7.2% | 18.8% | ||
| >11y | Count | 6 | 0 | 3 | 9 | |
| % within Time of after treatment (Year) | 66.7% | .0% | 33.3% | 100.0% | ||
| % within Type of treatment | 4.3% | .0% | 3.8% | 3.6% | ||
| % of Total | 2.4% | .0% | 1.2% | 3.6% | ||
| Total | Count | 140 | 32 | 78 | 250 | |
| % within Time of after treatment (Year) | 56.0% | 12.8% | 31.2% | 100.0% | ||
| % within Type of treatment | 100.0% | 100.0% | 100.0% | 100.0% | ||
| % of Total | 56.0% | 12.8% | 31.2% | 100.0% | ||
Table 11: Chi-Square Tests
| Value | df | Asymp. Sig. (2-sided) | |
| Pearson Chi-Square | 2.895(a) | 4 | 0.576 |
| Likelihood Ratio | 4.000 | 4 | 0.406 |
| Linear-by-Linear Association | 0.316 | 1 | 0.574 |
| N of Valid Cases | 250 |
a 2 cells (22.2%) have expected count less than 5. The minimum expected count is 1.15.
According to Chi-square test, a statistically significant association was observed between treatments with patients’ survival rate. (-0.57 = P)
In patients who have chemotherapy and radiotherapy treatment, thesurvival rate was 1-5 years in 56/ 7% and 51/1% survival rate was6-10 years and 66/7% survival rate was over 11 years. In patients who have chemotherapy in 14% of patients’ survival of 1-5 years and 10/6% survival was 10/6 percent.
In patients who have radiotherapy 29/4% survival rate was1-5 years, 38/3 survival rate was 6-10years, and at 3/33% survival rate was over 11 years.
Conclusions and recommendations
Results of examinations and tests carried out in compliance with the consequence studies are summarized as follows:
As in previous studies mentioned, tamoxifen reduces breast cancer recurrence in women with ER + and the increase in survival at 10 years period in our study the rate of recurrence of breast cancer in 250 patients was over a period of 10 year in 3 patients (1/2%).
As mentioned in previous studies, it was mentioned that the use of tamoxifen in women under 50 years is better, but in our study, no significant relationship between age at diagnosis and the patients after treatment was observed.
According to a study that was done in America, it was announced that treatment with tamoxifen increases the risk of endometrial cancer and thromboembolism. In this study, 250 patients treated with tamoxifen in women with breast cancer 2 (8/0%) had ovarian cancer.
In a study conducted in America in 1995, it was announced that the use of tamoxifen in a short time with an increased risk of cervical cancer is not associated with a reduced risk of breast of front side is that our study is consistent with it.
According to a study that was done in 1996 in Texas showed that tamoxifen works as an anti-estrogen in breast tissue, but estrogenic properties on bone, cardiovascular system and liver, and the women 2 to 3 times the general population are at risk for endometrial cancer. In this study, 1/2% of patients with bone metastases and 0/8%of patients had liver cancer.
The results of this study and other studies of tamoxifenis effective along with other treatments in controlling disease in particular, this study suggests that more research is done in this regard.
Also according to the cancer cells are deprived from the estrogen hormone supplement treatment which some breast cancer cells need to grow and according to the study and research on older samples of Research Center was shown when tamoxifen prescribed as adjuvant treatment for early-stage breast cancer to prevent the recurrence of cancer and prevention of new cancers in the other breast.
Therefore, removal of the ovaries in premenopausal women instead of tamoxifen is recommended.
Although, there was no significant association between age at diagnosis and survival but according to surveys conducted in 250 patients showed lowerage disease is an alarm. And the worrying thing about this is mentioned in the study more than 50% of cancer before the age of 50, respectively.Due to the fact and the results from this study that was effective treatment based on diagnosis of lower stage associated with lower treatment Initiating screening at an early age are much lower than current standard of care and follow-up.
References
- Nananda F, Robert J, Richard K. Survival impact of tamoxifen use for cancer risk reduction: projection from a patient-specific marker model. Society fos medical Decision making, 2002.
- Coombers RC, Kilburn LS, Snowdan CF. Survival and safety of versus tamoxifen after 2-3 years (inter group exemestone study and randomized controlled trial. National Library of medicine national institute of health, 2007.
- Nobhot Z, Buzdar M, Pellak W. Tamoxifen for early breast cancer and overview of the randomized trials, early breast cancer trialists collaborative group. The Journal of Onchology, 1998.
- Hershman D, Daniel F, Alfred I. Outcomes of tamoxifen chemopreve for breast cancer in very high risk women a coast effectiveness analysis. Journal of Onchology, 2002.
- Buzdar AV. Tamoxifen clinical application: old and new. US National Library of medicin, 2000.
- Fisher B, Dignam J, Emir B. Tamoxifen and chemotherapy for lymph node, negative strogen, positive breast cancer. Journal of the national cancer Institute, 1997.
- Slomovitz, Brain M, Chattote C. Dose tamoxifen use affect prognosis in breast cancer patients who develop endometrial cancer. The American Collage of abstetricans and Gynacologists, 2004.
- Crabb WW. The tamoxifen controversy. US national library of medicine, 1996.
- Linda S, Noel S, Stephan M. Population based study of tamoxifen therapy and subsequent ovarian, endometrial and breast cancer. Journal of the national cancer Institute, 1995.
- Jiang, Massarve S, Kent Asborn C. Mechanism of tamoxifen resistance, increase estrogen HER2/Neu cross talk in ER/HER2 positive breast cancer. INCI, 2004.








