Divya Sasitharan1, M. Masilamani Selvam1, Manohar Paul2
1Department of Biotechnology, Sathyabama University, Chennai, India 2Department of Microbiology,Tamil Nadu University of Veterinary and Animal Sciences,Chennai, India
DOI : https://dx.doi.org/10.13005/bpj/619
Abstract
To compare the efficacy of a whole-cell vaccine and a toxoid vaccine prepared for Escherichia coli O157:H7 strain using rats as animal models after pre-clinical methods of testing. Methods: Isolation and identification of study strain from samples such as ground beef, chicken intestine, raw milk and pasteurized milk was followed by confirmation of five selected virulence genes by conventional PCR amplification. Whole cell vaccine and a toxoid vaccine, prepared as protein produced out of stx1 gene variant from an isolate strain of study were subjected to its efficacy test using bioinformatics tools, cell culture technique and clinical tests using rat models after obtaining proper ethical clearance. After challenging with ATCC and isolates, screening performed by histology in liver and kidney on test rats as well as on control rats. Results: Cytopathic effects of prepared vaccines in vero cell lines and SavesV4 software results showed loss of pathogenicity and retainment of immunogenicity of the study strain. Normal histology was seen in liver and kidney specimens of vaccinated and challenged rat groups as well as control un-inoculated unvaccinated rat groups with very few exceptional lesions. However, severe toxic evidences were observed in rat groups which were unvaccinated and challenged with pathogenic strains. Conclusions: Vaccine is believed to have lost its pathogenicity to a greater extent as validated by pre-clinical and animal studies. When comparison was made between whole cell vaccine and toxoid, whole cell vaccine had greater extent of efficiency than toxoid.
Keywords
Escherichia coli O157:H7; Toxoid Vaccine; Whole-cell vaccine; Haemolytic Uremic Syndrome
Download this article as:| Copy the following to cite this article: Sasitharan D, Selvam M. M, Paul M. Pre-Clinical and Clinical Validation of Vaccines against Hemolytic Uremic Syndrome. Biomed Pharmacol J 2015;8(1) |
| Copy the following to cite this URL: Sasitharan D, Selvam M. M, Paul M. Pre-Clinical and Clinical Validation of Vaccines against Hemolytic Uremic Syndrome. Biomed Pharmacol J 2015;8(1). Available from: http://biomedpharmajournal.org/?p=762 |
Introduction
Vaccine regimens which mimic actual infection offer the best opportunity for successful long-term immunoprotection against many bacterial diseases. Preclinical as well as clinical studies and testing strategies have the purpose of limiting risks, evaluating safety and immunogenicity, whenever a vaccine is to be used in humans. This Escherichia coli O157:H7 is an enterohemorrhagic strain of the bacterium Escherichia coli and a cause of food-borne illness. Most illness has been associated with eating undercooked, contaminated ground beef, drinking unpasteurized milk, swimming in or drinking contaminated water, and eating contaminated vegetables. Young children and females had an increased risk of Haemolytic uremic syndrome (HUS) after Shiga-like Toxic producing Escherichia coli (STEC) O157 infection. With or without HUS, elderly persons had the highest proportion of deaths associated with STEC O157 infection. These data support recommendations for aggressive supportive care of young children and the elderly early during illness due to STEC O157 (Gould L.H. et. al., 2009). HUS is characterised by three features-acute renal failure, microangiopathic haemolytic anaemia and thrombocytopenia (Levin M. and Barratt J.M., 1984). A close association between VTEC and HUS was first reported by Karmali M. A., et al. in 1983 and studies since then have established that VTEC are a major cause of the typical form of HUS (Karmali M. A., et al. in 1983 and Scotland S.M., et. al., 1988). Escherichia coli O157:H7 was the causative agent of many out-breaks worldwide.
Outbreaks of HC caused by VTEC occurred in different areas of the USA in 1982 (Johnson W. M. et. al., 1983) and since then outbreaks and sporadic cases have been reported in several other countries including Canada, Britain and Japan (Pai C.H. et al., 1984, Itoh T, 1985 and Smith H. R. et. al., 1987). Incidence of sporadic cases of hemorrhagic colitis due to Escherichia coliO157:H7 may be higher than suspected (Chik H. Pai. et al., 1984). Hence, an epidemiological outbreak of Escherichia coli O157:H7 can be expected in near future and a constant prevalent study has to be conducted throughout the world
Aim and Obective
To test a whole-cell vaccine and a toxoid (inactivated stx1 toxin) produced from Escherichia coli O157:H7, for having its pathogenicity lost, pre-clinically using bioinformatics tools and cell culture techniques and hence, by clinical methods using wistar rat models
Materials and Methods
A. Isolation and Identification of Strains
Around 500 samples were collected in a 2 years’ time from five different sources such as small intestine of chicken, ground beef, cattle faeces, raw milk and pasteurized milk, and brought to the laboratory with standard care. ATCC strain was obtained from Madras Veterinary College, Chennai, India. Identification of Escherichia coli O157 was done by performing the routine microbiological tests, such as Gram staining, motility, catalase test, oxidase test, carbohydrate fermentation and culture characterization. Also, latex agglutination test for confirmation of O157 strain was done by using “LK13 HiE.coliTM 157 Latex Test Kit”. Apart from general characterization, antibiotic sensitivity tests were performed to determine antimicrobial resistance profiles.
B. Genotypic confirmation
Conventional PCR amplification confirmed the presence of five selected virulence genes, namely Intimin (eae), Shigatoxin1 (stx)1, Shigatoxin2 (stx2), Hemolysin (hlyA) and Flagellar antigen (fliCh7) genes. Oligonucleotide primer sequences used for PCR amplification was derived from a study conducted by EL-Jakee J. et al., in Egypt in 2009.
C. Toxoid Vaccine Preparation
A protein vaccine was produced from genetically modified gene of Escherichia coli O157:H7. Stx1 gene from Escherichia coli O157:H7 was PCR amplified and sequenced. Cloned in E.coli BL21 strain using TA vector. Cloned in Expression vector using LIC alicator kit and proteins were expressed. Expressed proteins were purified using Ni-Nta column and confirmed by SDS-PAGE. Inactivated toxin produced by altering stx1 gene (restriction digested by selected duet restriction site oriented enzyme, namely Xho1 enzyme), which was also cloned and expressed similar to cloned stx1 gene.
D. Whole-Cell Vaccine Preparation
A whole cell formalin killed vaccine was produced out of Escherichia coli O157:H7. Jan Holmgren et al., 2003, disclosed a method for producing a formalin-killed E. coli bacterial strain for use in a vaccine against enteric infection caused by E. coli bacteria in humans. E. coli bacterial strain was grown in liquid culture medium with vigorous agitation to a predetermined density, E. coli bacterial strain was harvested, and harvested E. coli bacterial strain was resuspended in saline. Formalin added to harvested, resuspended bacterial strain to a final concentration of 0.2M formaldehyde. Incubated at 37 C under conditions of continuous agitation for about 2 hours, further incubated formalin-treated bacterial strain at 4 C for about 24-48 hours, thereby obtaining a formalin-killed E. coli bacterial strain and, collecting said formalin-killed E. coli bacterial strain. Prepared vaccine was subjected to its efficacy test.
A. Pre-Clinical Validation of Toxoid and Whole-Cell Vaccine by Cell Culture Technique and Bioinformatic tools
Cultured vero cell lines were infected with the purified protein (stx1 toxin) and CPE observed. Inactivated toxin produced by altering stx1 gene was also cloned and expressed similar to cloned stx1 gene. Hence, purified and infected in vero cells too. Cytopathic effects shown by stx1 toxin when infected in vero cell lines were shown to have lost its pathogenicity when compared with stx1 toxoid infected vero cell lines (Divya Sasitharan et. al., 2013). Further, modelling the genes (before and after alterations) using swiss-model software and validation by Saves v4 software tools proved the inactivation of active site of toxin at in-vitro level (Divya Sasitharan et. al., 2013). These vaccines were further subjected to its in-vivo efficacy test using laboratory animals.
B. Clinical Validation of Toxoid and Whole-Cell Vaccine by Animal Studies
Ethical clearance was obtained for performing clinical trials using animal models. Ethical clearance numbered KMCRET/PhD/07/2013-14 was obtained from KMCH College of Pharmacy, Coimbatore, as approved on 5th April 2013. Experimental design for clinical studies involving wistar rat models is depicted in Figure 1, which included a total of seven groups as: Three control groups of wistar rats (1 group for complete uninoculated control, 1 group for ATCC and 1 group animal for isolate stain administration) and two test group rats (1 group for ATCC strain challenging after whole cell vaccination and 1 group for isolated strain challenging after whole cell vaccinating) and two test group rats (1 group for ATCC strain challenging after toxoid vaccination and 1 group for isolated strain challenging after toxoid vaccinating). Vaccine doses were given by oral route as per protocol of Evans D. J. et al., 1988. Screening methods emphasised on studying the effects on test rats as well as on control rats (mortality as well as morbidity in liver and kidney with the help of histopathology).
Results and discussion
The morphological (gram staining and motility test), sugar fermentation (glucose, sucrose, lactose, and maltose), biochemical (indole, methyl red, voges-proskauer, citrate, urease, catalase, oxidase) characterization of Escherichia coli O157:H7 isolate as well as ATCC were performed. The colony morphology of Escherichia coli O157:H7 isolates as well as ATCC strain, when grown on various medium like Nutrient agar, Eosin methylene blue agar, MacConkey agar and MacConkey Sorbitol agar base with added tellurite, was observed. Latex agglutination test was also done for confirmation of the strain using “LK13 HiE.coliTM 157 Latex Test Kit”. It was observed that more than 90% of samples were positive for Escherichia coli but only 5 samples were positive for the bacteria Escherichia coli O157:H7. The antibiotic patterns, the multiple drug resistance and the frequency of antibiotic resistance exhibited by the five isolates of Escherichia coli O157:H7 and ATCC strain were also observed and compared. A cent percent resistance was observed for Ampicillin, Amoxicillin, and Tetracycline. Genotypic confirmation was done with conventional PCR amplification in all five isolates and prevalence of 5 different virulence genes was determined.
Cell Culture Effects and Bioinformatics Results
Cytopathic effects were highly shown by stx1 toxoid but not seen with stx1 toxin when infected in vero cell lines. Validation of stx1 toxin passed while stx1 toxoid model failed in it’s A-subunit using prove, verify 3D, procheck and whatcheck tools of SavesV4 software. Moreover, in both cases B-subunit got through. This shows loss of pathogenicity and retainment of immunogenicity of the study strain.
Histopathology For Vaccine Testing
Reports revealed the following:
1) In Rat Group No. 1 (Neither vaccine nor strain administered), Group No. 4 (Vaccinated with prepared toxoid vaccine and challenged with ATCC strain), Group No. 5 (Vaccinated with prepared toxoid vaccine and challenged with Isolate strain), Group No. 6 (Vaccinated with prepared whole-cell vaccine and challenged with ATCC strain), and Group No. 7 (Vaccinated with prepared whole-cell vaccine and challenged with Isolate strain): Liver and Kidney Specimen [As shown in Figure 2]: Normal lobular architecture, portal tracts, central vein and sinusoids. The hepatic parenchyma is unremarkable. No inflammation, toxic changes or fibrosis. Normal cortex, medulla, pelvicalyceal system and distal convoluted tubules. Cortex shows normal glomeruli, proximal convoluted tubules. The interstitium is unremarkable. No evidence of toxic changes.
2) In Rat Group No. 2 (Unvaccinated and ATCC administered) and Group No.3 (Unvaccinated and Isolate strain administered): Liver and Kidney Specimen [As shown in Figure 3]: Intact architecture with lobular inflammation. The portal tracts show acute inflammation and mild lymphocytic infiltration. The hepatic parenchyma shows lobular inflammation with focal granuloma is noted. The central vein and sinusoids are mildly dilated. Some of the glomeruli with loss of shape with increased in interstitium indicating questionable fibrosis. The proximal convoluted tubules show dilatation and shedding of epithelium indicating tubular necrosis, in some areas. The distal convoluted tubules are markedly dilated at places. Interstitium shows lymphocytic inflammation. Few congested blood vessels also noted.
To summarise the results, vaccinated rats showed normal pathology as that of unchallenged control group and unvaccinated rat models showed abnormal lesions. However, negligible exceptional lesions were seen in toxoid vaccinated rats after challenging with pathogen whereas whole-cell vaccinated rats showed cent percent protection when challenged with pathogen.
 |
Figure 1: Experimental Design of Animal Studies Work. |
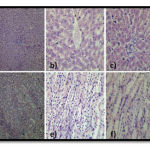 |
Figure 2
|
Figure 2. Histology of Liver and Kidney Specimens of Group 1, 4, 5, 6 and 7: Normal lobular architecture, portal tracts, central vein and sinusoids. The hepatic parenchyma is unremarkable. No inflammation, toxic changes or fibrosis. a) 10X shows normal lobular architecture. b) 40X shows normal central vein. c) 40X shows normal portal tract. Normal cortex, medulla, pelvicalyceal system and distal convoluted tubules. Cortex shows normal glomeruli, proximal convoluted tubules. The interstitium is unremarkable. No evidence of toxic changes. d) 10X shows normal glomeruli and tubules. f) 40X shows normal interstitium. g) 40X shows normal tubules.
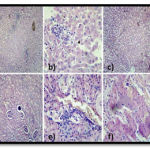 |
Figure 3 |
Figure 3. Histology of Liver and Kidney Specimens of Group 2 and 3: Intact architecture with lobular inflammation. The portal tracts show acute inflammation and mild lymphocytic infiltration. The hepatic parenchyma shows lobular inflammation with focal granuloma is noted. The central vein and sinusoids are mildly dilated. a) 10 x shows normal lobular architecture with lobular inflammation. b) 40 x shows dilated sinusoids. Some of the glomeruli with loss of shape with increased in interstitium indicating questionable fibrosis. The proximal convoluted tubules show dilatation and shedding of epithelium indicating tubular necrosis, in some areas. The distal concoluted tubules are markedly dilated at places. Interstitium shows lymphocytic inflammation. Few congested blood vessels also noted. c) 10X shows dilated tubules with congested blood vessels. d) 10X shows glomeruli with degenerative changes and focal inflammation. e) 40X shows interstitium with congestion. f) 40X shows shedding of epithelium with dilated tubules
Conclusion
So far, no toxoid vaccine has been prepared worldwide for Escherichia coli O157:H7, hence no comparison could be made with literature studies. When comparison was made with efficiency of two types of prepared vaccines in this study, namely toxoid and whole-cell vaccine, both were believed to have lost its pathogenicity as validated by pre-clinical in-vitro studies and clinical animal studies but whole-cell vaccine has proved to show much higher efficiency than toxoid vaccine. However, this is just a preliminary confirmation before proceeding to human volunteer level studies.
References
- Chik H. Pai, R. T. Rhonda Gordon, Harry V. Sims and Lawrence E. Bryan, “Sporadic cases of hemorrhagic colitis associated with Escherichia coli 0157:H7: Clinical, epidemiologic, and bacteriologic features”, Annual Internal Medicine, 1984, 101(6): 738-742.
- Divya Sasitharan, Masilamani Selvam M. and Manohar Paul W., 2013. ICETCST editors. titled “Preclinical validation of toxoid vaccine by cell culture approach” in the Proceedings of the International Conference on Emerging Trends and Challenges in Science and Technology – ICETCST; 2013 March 1-2; Chennai, India; Margham Publications, pp 334-337.
- Divya Sasitharan, Masilamani Selvam M., Manohar Paul W., 2013. Conference paper titled “Preclinical validation of toxoid vaccine by bioinformatics approach” presented at the National Seminar on Emerging Trends in Microbial Biotechnology, Kanchi Krishna College, Kancheepuram, India, 20-21 February 2013.
- J. EL-Jakee, E. I. Moussa, Mohamed KhF. and G. Mohamed, “Using molecular techniques for characterization of Escherichia coli isolated from water sources in Egypt”, Global Veterinaria, 2009, 3(5): 354-362.
- D. J. Evans Jr., D. G. Evans, A. R. Opekun, D. Y. Graham, “ Immunoprotective oral whole cell vaccine for enterotoxigenic Escherichia coli diarrhea prepared by in situ destruction of chromosomal and plasmid DNA with colicin E2”, FEMS Microbiol Immunol, 1988, 1(1):9-18.
- L. H. Gould, L. Demma, T. F. Jones, S. Hurd, D. J. Vugia, K. Smith, B. Shiferaw, S. Segler, A. Palmer, S. Zansky and P. M. Griffin, “Hemolytic uremic syndrome and death in persons with Escherichia coli O157:H7 infection, food-borne diseases active surveillance network sites, 2000-2006”, Clin Infectious Diseases, 2009, 15(10): 1480-1485.
- T. Itoh, “Epidemiological and laboratory investigation of an outbreak of acute enteritis associated with cytotoxin producing Escherichia coli O145:H7”, Annual Report of Tokyo Metropolitan Research Laboratory for Public Health, 1985, 36: 16-22.
- Jan Holmgren and Ann-Mari Svennerholm., “Preparation and use of formalin-killed colonization-factor-antigen (CFA)-Expressing E.coli organisms for vaccination against enteric infection/diarrhae caused by enterotoxigenic E.coli bacteria in humans”, Patent No-US 6558678 B1, obtained on 6th May 2003.
- W. M. Johnson, H. Lior and G. S. Bezanson, “Cytotoxic Escherichia coli O157: H7 associated with haemorrhagic colitis in Canada”, Lancet, 1983, 1: 76.
- M. A. Karmali, M. Petric, C. Lim, P. C. Fleming, G. S. Arbus and H. Lior, “The association between idiopathic haemolytic uremic syndrome and infection by Verotoxin-producing Escherichia coli”, Journal of Infectious Diseases, 1985, 151: 775-782.
- M. A. Karmali, B. T. Steele, M. Petric and C. Lim, “Sporadic cases of haemolytic-uraemic syndrome associated with faecal cytotoxin and cytotoxin-producing Escherichia coli in stools”, Lancet, 1983, 1: 619-620.
- M. Levin and J. M. Barratt, “Haemolytic uraemic syndrome”, Archives of Disease in Childhood, 1984, 59: 397-400.
- C. H. Pai, R. Gordon, H. V. Sims and L. E. Bryan, ”Sporadic cases of hemorrhagic colitis associated with Escherichia coli O157:H7: Clinical, epidemiologic, and bacteriologic features. Annuals of Internal Medicine, 1984, 101: 738-742.
- S. M. Scotland, B. Rowe, H. R. Smith, G. A. Willshaw and R. J. Gross, “Vero cytotoxin-producing strains of Escherichia coli from children with haemolytic uraemic syndrome and their detection by specific DNA probes”, Journal of Medical Microbiology, 1988, 25: 237-243.
- H. R. Smith, B. Rowe, R. J. Gross, N. K. Fry and S. M. Scotland, “Haemorrhagic colitis and Vero cytotoxin-producing Escherichia coli in England and Wales”, Lancet, 1987, 1: 1062-1064.








