Manuscript accepted on :
Published online on: 21-11-2015
Plagiarism Check: Yes
1Lauro Figueroa-Valverde*, 3Marcela Rosas-Nexticapa, 2Francisco Díaz-Cedillo, 1Lenin Hau-Heredia, 1Elodia García-Cervera, 1Eduardo Pool-Gómez1, 4Rolando García-Martínez, and 5Abelardo amacho-Luis.
1Laboratory of Pharmacochemistry, Faculty of Chemical and Biological Sciences; University Autonomous of Campeche. Av. Agustín Melgar, Col Buenavista C.P. 24039, Campeche Cam., México. 2Escuela Nacional de Ciencias Biológicas del Instituto Politécnico Nacional. Prol. Carpio y Plan de Ayala s/n Col. Santo Tomas, México, D.F. C.P. 11340. 3Facultad de Nutrición, Universidad Veracruzana. Médicos y Odontólogos s/n, 91010, Xalapa, Veracruz, México. 4Laboratorio de Neurociencias del Centro de Investigaciones Biomédicas de la Universidad Autónoma de Campeche. Av. Patricio Trueba y Regil s/n, Col. Lindavista C.P. 24090, Campeche Cam., México. 5Facultad de Medicina y Nutrición, Centro Investigación en alimentos y nutrición. Universidad Juárez del Estado de Durango. Av. Universidad s/n esq. Fanny Anitua, Zona centro. Durango, México.
DOI : https://dx.doi.org/10.13005/bpj/600
Abstract
There is scarce information about the effects exerted by the naphthalene derivatives on cardiac injury caused by ischemia/reperfusion. In this study, a new naphthalene derivative was synthetized with the objective of evaluating its activity on ischemia/reperfusion injury. The Langendorff technique was used to evaluate the effect of the naphthalene derivative on ischemia/reperfusion injury. Additionally, molecular mechanism involved in the activity exerted by the naphthalene derivative on perfusion pressure and coronary resistance was evaluated by measuring left ventricular pressure in absence or presence of following compounds; propanolol, prazosin, metoprolol, indomethacin and nifedipine. The results showed that the naphthalene derivative reduces infarct size compared with control. Other results showed that the naphthalene derivative significantly increase (p = 0.05) the perfusion pressure and coronary resistance in isolated heart. In addition, other data indicate that the naphthalene derivative increase left ventricular pressure in a dose-dependent manner (0.001 to 100 nM); however, this phenomenon was significantly inhibited by nifedipine at a dose of 1 nM (p = 0.05). In conclusion, these data suggest that naphthalene derivative exert a cardioprotective effect via the calcium channels activation and consequently induces changes in the left ventricular pressure levels. This phenomenon results in decrease of myocardial necrosis after ischemia and reperfusion.
Keywords
Naphthalene; left ventricular pressure; nifedipine
Download this article as:| Copy the following to cite this article: Rosas-Nexticapa M, Figueroa-Valverde L, Díaz-Cedillo F, Hau-Heredia L, García-Cervera E, Pool-Gómez E, García-Martínez R, Amacho-Luis A. New Naphthalene-Derivative as Cardioprotector Drug on the Ischemia/Reperfusion Injury. Biomed Pharmacol J 2015;8(1) |
| Copy the following to cite this URL: Rosas-Nexticapa M, Figueroa-Valverde L, Díaz-Cedillo F, Hau-Heredia L, García-Cervera E, Pool-Gómez E, García-Martínez R, Amacho-Luis A. New Naphthalene-Derivative as Cardioprotector Drug on the Ischemia/Reperfusion Injury. Biomed Pharmacol J 2015;8(1). Available from: http://biomedpharmajournal.org/?p=1224 |
Introduction
There are several data clinical and epidemiological which indicate that myocardial infarction is a main cause of death worldwide [1,2]. Myocardial infarction can be induced by prolonged ischemia which consequently brings in cell viability, and ultimately cardiac function. In addition, acute myocardial infarction can produce alterations in the topography of both the infarcted and non-infarcted regions of the ventricle [3]. Some reports which indicate the most effective method of limiting necrosis is restoration of blood flow; however, the effects of reperfusion itself may also be associated with tissue injury [4]. In the search of therapeutic alternatives to reduce the ischemia-reperfusion injury, some drugs have been evaluated; for example, there are data which indicate that glibenclamide reduces myocardial damage caused by ischemia/reperfusion in Guinea pig heart via activation of ATP-regulated K+ channels [5]. Other study indicates that Cyclosporin A can reduce the ischemia/reperfusion-injury in isolated rat hearts through changes in cAMP levels [6]. In addition, some naphthalene derivatives have been developed to evaluate their biological activity in several animal models; for example, a study showed that the naphthalene derivative ((±)-(R,S)-5,6-dihydroxy-2-methylamino-1,2,3,4-tetrahydro-naphthalene hydro-chloride) induces protective effects on ischemia-reperfusion injury in isolated heart rat via decrease of norepinephrine [7]. Other report, showed that the naphthalene derivative (N-(6-aminohexyl)-5-chloro-1-naphthalene sulfonamide), exerts cardioprotective effects on the isolated rat heart exposed to hypothermic and ischemic conditions by blocking the activity of calmodulin [8]. Other data indicates that compound 1,5-(dimethylamino)-N-(3,4-dimethyl-5-isoxazolyl)-1-naphthalene sulfonamide, significantly improved the recovery of cardiac function during reperfusion-ischemia injury through of reduced of creatine kinase [9]. All these data indicates that differences in the chemical structure of naphthalene derivatives may be in part responsible of activity of these compounds on reperfusion-ischemia injury. To test this information, the present study was designed to investigate the effects induced by a naphthalene derivative in a myocardial infarction/reperfusion model. In addition, to evaluate the molecular mechanism involved in the activity of the naphthalene derivative on left ventricular pressure which were used as pharmacological tools for blocking various biological systems; propanolol (non-selective β-receptor blocker) [10], prazosin (α1 adrenoreceptor antagonist) [11], metoprolol (selective β1 receptor blocker) [12], indomethacin (prostaglandin synthesis inhibitor) [13] and nifedipine (antagonist calcium channel type L) [14].
Material and Methods
Chemical synthesis
The compounds evaluated in this study were purchased from Sigma-Aldrich Co., Ltd. The melting point for the danazol derivative was determined on an Electrothermal (900 model). Infrared spectra (IR) were recorded using KBr pellets on a Perkin Elmer Lambda 40 spectrometer. 1H and 13C NMR (nuclear magnetic resonance) spectra were recorded on a Varian VXR-300/5 FT NMR spectrometer at 300 and 75.4 MHz (megahertz) in CDCl3 (deuterated chloform) using TMS (tetramethylsilane) as internal standard. EIMS (electron impact mass spectroscopy) spectra were obtained with a Finnigan Trace Gas Chromatography Polaris Q Spectrometer. Elementary analysis data were acquired from a Perkin Elmer Ser. II CHNS/0 2400 elemental analyzer.
Synthesis of 2-hydroxy-N-(4-nitrobenzoyl)naphthalene-1-carboximidic acid
A solution of 4-nitrobenzoyl azide (100 mg, 0.52 mmol), 2-hydroxy-1-naphthaldehyde (90 mg, 0.52mmol) and acetic acid (3 mL) in 10 mL of dimethylsulfoxide/methanol (3:1) was stirred for 24 h at room temperature. The reaction mixture was evaporated to dryness under reduced pressure, the residue washed 3 times with water. Then the precipitate was separated and dried at room temperature.
Biological method
All experimental procedures and protocols used in this investigation were reviewed and approved by the Animal care and use Committee of University Autonomous of Campeche (No. PI-420/12) and were in accordance with the guide for the care and use of laboratory animals [15]. Male Wistar rats; weighing 200-250 g were obtained from University Autonomous of Campeche.
Reagents
All drugs were dissolved in methanol and different dilutions were obtained using Krebs-Henseleit solution (≤ 0.01%, v/v).
Experimental design
Briefly, the male rat (200 – 250 g) was anesthetized by injecting them with pentobarbital at a dose rate of 50 mg/Kg body weight. Then the chest was opened, and a loose ligature passed through the ascending aorta. The heart was then rapidly removed and immersed in ice cold physiologic saline solution. The heart was trimmed of non-cardiac tissue and retrograde perfused via a non-circulating perfusion system at a constant flow rate. The perfusion medium was the Krebs-Henseleit solution (pH = 7.4, 37°C) composed of (mmol); 117.8 NaCl; 6 KCl; 1.75 CaCl2; 1.2 NaH2PO4; 1.2 MgSO4; 24.2 NaHCO3; 5 glucose and 5 sodium pyruvate. The solution was actively bubbled with a mixture of O2/CO2 (95:5/5 %). The coronary flow was adjusted with a variable speed peristaltic pump. An initial perfusion rate of 15 ml/min for 5 min was followed by a 15 min equilibration period at a perfusion rate of 10 ml/min. All experimental measurements were done after this equilibration period.
Perfusion pressure
Evaluation of measurements of perfusion pressure changes induced by drugs administration in this study were assessed using a pressure transducer connected to the chamber where the hearts were mounted and the results entered into a computerized data capture system (Biopac).
Inotropic activity
Contractile function was assessed by measuring left ventricular developed pressure (LV/dP), using a saline-filled latex balloon (0.01 mm, diameter) inserted into the left ventricle via the left atrium [16]. The latex balloon was bound to cannula which was linked to pressure transducer that was connected with the MP100 data acquisition system.
First stage
Ischemia/Reperfusion model
After of 15-minute equilibration time, the hearts were subjected to ischemia for 30 minutes by turning off the perfusion system [17]. After this period, the system was restarted and the hearts were reperfused by 30 minutes with Krebs-Henseleit solution. The hearts were randomly divided into 2 major treatment groups with n = 9:
Group I. Hearts were subjected to ischemia/reperfusion but received vehicle only (Krebs- Henseleit solution).
Group II. Hearts were subjected to ischemia/reperfusion and treated with the naphthalene derivative (0.001 nM) before ischemia period (for 10 minutes) and during the entire period of reperfusion. At the end of each experiment, the perfusion pump was stopped, and 0.5 ml of fluorescein solution (0.10%) was injected slowly through a sidearm port connected to the aortic cannula. The dye was passed through the heart for 10 sec to ensure its uniform tissue distribution. The presence of fluorescein was used to demarcate the tissue that was not subjected to regional ischemia, as opposed to the risk region. The heart was removed from the perfusion apparatus and cut into two transverse sections at right angles to the vertical axis. The right ventricle, apex, and atrial tissue were discarded. The areas of the normal left ventricle non risk region, area at risk, and infarct region were determined using methods previously reported [18]. Total area at risk was expressed as the percentage of the left ventricle.
Second stage
Effect induced by the compounds 4-nitrobenzoyl azide, 2-hydroxy-1-naphthaldehyde and the naphthalene derivative on perfusion pressure:
Changes in perfusion pressure as a consequence of increases in time (3 to 18 min) in absence (control) or presence of the compounds 4-nitrobenzoyl azide, 2-hydroxy-1-naphthaldehyde and the naphthalene derivative at a concentration of 0.001 nM were determined. The effects were obtained in isolated hearts perfused at a constant-flow rate of 10 ml/min.
Evaluation of effects exerted by the compounds 4-nitrobenzoyl azide, 2-hydroxy-1-naphthaldehyde and the naphthalene derivative on coronary resistance
The coronary resistance in absence (control) or presence of the compounds 4-nitrobenzoyl azide, 2-hydroxy-1-naphthaldehyde and the naphthalene derivative at a concentration of 0.001 nM was evaluated. The effects were obtained in isolated hearts perfused at a constant flow rate of 10 ml/min. Since a constant flow was used changes in coronary pressure reflected the changes in coronary resistance.
Third stage
Effects induced by the naphthalene derivative on left ventricular pressure through α1– adrenergic receptor.
Intracoronary boluses (50 μl) of the naphthalene derivative (0.001 to 100 nM) were administered and the corresponding effect on the left ventricular pressure was determined. The dose-response curve (control) was repeated in the presence of prazosin at a concentration of 1 nM (duration of preincubation with prazosin was by a 10 min equilibration period).
Effects induced by the naphthalene derivative on left ventricular pressure through β1– adrenergic receptor.
Intracoronary boluses (50 μl) of the naphthalene derivative (0.001 to 100 nM) were administered and the corresponding effect on the left ventricular pressure was determined. The dose-response curve (control) was repeated in the presence of propranolol or metoprolol at a concentration of 1 nM (duration of preincubation with propanolol or metoprolol was by a 10 min equilibration period).
Effect exerted by the naphthalene derivative on left ventricular pressure in the presence of indomethacin.
The boluses (50 μl) of the naphthalene derivative [0.001 to 100 nM] were administered and the corresponding effect on the left ventricular pressure was evaluated. The bolus injection administered was done in the point of cannulation. The dose response curve (control) was repeated in the presence of indomethacin at a concentration of 1 nM (duration of the pre-incubation with indomethacin was for a period of 10 min).
Effects of the naphthalene derivative on left ventricular pressure through the calcium channel
Intracoronary boluses (50 μl) of the naphthalene derivative [0.001 to 100 nM] were administered and the corresponding effect on the left ventricular pressure was evaluated. The dose-response curve (control) was repeated in the presence of nifedipine at a concentration of 1 nM (duration of the pre-incubation with nifedipine was for a period of 10 min).
Effects induced by the naphthalene derivative on the intracellular calcium levels
Changes in calcium levels as a consequence of increases in time (3-18 min) induce by the naphthalene derivative at a dose of 0.001 nM (dose minimum) were evaluated. The dose-response curve (control) was repeated in the presence of nifedipine at a concentration of 1 nM mmol (duration of preincubation with nifedipine was by a 10 min equilibration period). It is important to mention, that intracellular calcium was evaluated using previously methods reported [18]. In this method, 5 mL of samples obtained of perfused (metabolic solution) were used to evaluate the intracellular calcium. It is important to mention that the samples were taken every 3 min (six times). After, 4 ml trifluoroacetic acid (10%) was added each to sample. This solution was mixed for 5 min at room temperature and after 1 ml of NaOH (25%) was added. To mixture 1 ml was trisodic phosphate added and the mixture was vortexed (1 min) and centrifuged to 4000 rpm (5 min). The supernatant was separated from the aqueous solution. After 5 mL of a buffer solution (pH = 10) and 3 ml of eriochromeblack was added to the aqueous solution. The mixture was titled with ethylenediaminetetraaceticacid (EDTA) (f = 0.847).
Statistical analysis
The obtained values are expressed as average ± SE, using each heart (n = 9) as its own control. The data obtained were put under Analysis of Variance (ANOVA) with the Bonferroni correction factor using the SPSS 12.0 program [19]. The differences were considered significant when p was equal or smaller than 0.05.
Results
Chemical synthesis
The yield of the reaction product (compound 3, Figure 1) was 45 % with melting point of 188-190 °C. In addition, the spectroscopic analyses show signals for IR (Vmax, cm-1) at 3400, 1280. In addition, the chemical shifts of the spectroscopic analyses of 1H NMR and 13C NMR for the steroid derivative is showed in the table 2 and 3. Finally, the results of mass spectroscopy (MS) (70 electrovolts) shown; m/z 830.52. Additionally, the elementary analysis data for the steroid derivative (C18H12N25) were calculated (C, 64.29; H, 3.60; N, 8.33; O, 23.79) and found (C, 64.20; H, 3.54).
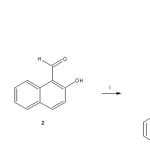 |
Figure 1: Synthesis of 2-hydroxy-N-(4-nitrobenzoyl)naphthalene-1-carboximidic acid (3). Reaction of 4-nitrobenzoyl azide (1) with 2-hydroxy-1-naphthaldehyde (2) to form the compound 3. i = acetic acid. |
Statistical Analysis
The obtained values are expressed as average ± SE, using each heart (n = 9) as its own control. The data obtained were put under Analysis of Variance (ANOVA) with the Bonferroni correction factor using the SPSS 12.0 program [19]. The differences were considered significant when p was equal or smaller than 0.05.
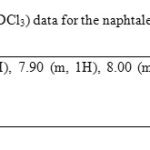 |
Table 1 |
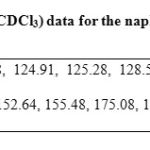 |
Table 2: 13C NMR (75.4 MHz, CDCl3) data for the naphtalene derivative. |
Results
Chemical synthesis
The yield of the reaction product (compound 3, Figure 1) was 45 % with melting point of 188-190 °C. In addition, the spectroscopic analyses show signals for IR (Vmax, cm-1) at 3400, 1280. In addition, the chemical shifts of the spectroscopic analyses of 1H NMR and 13C NMR for the steroid derivative is showed in the table 2 and 3. Finally, the results of mass spectroscopy (MS) (70 electrovolts) shown; m/z 830.52. Additionally, the elementary analysis data for the steroid derivative (C18H12N25) were calculated (C, 64.29; H, 3.60; N, 8.33; O, 23.79) and found (C, 64.20; H, 3.54).
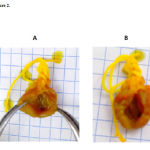 |
Figure 2: Comparison of cardioprotective effect of the naphtalene derivative (B) at a dose of 1 nM with the control (A) on the functional recovery of rat hearts subjected to ischemia and reperfusion. |
 |
Figure 3: Effect exerted by the naphthalene derivative on ischemia-reperfusion injury. The results showed that the naphthalene derivative significantly reduced infarct size expressed as a percentage of the area at risk compared with the vehicle-treated hearts (p = 0.05). Each bar represents the mean ± S.E. of 9 experiments. N-D = naphthalene derivative (com- pound 3). |
Second stage
The activity induced by estradiol and its derivatives on perfusion pressure and coronary resistance in the isolated rat hearts was evaluated. The results (Figure 4) showed that the naphthalene derivative (0.001 nM) significantly increase the perfusion pressure (p = 0.05) over time (3-18 min) compared with the control conditions, 4-nitrobenzoyl azide and 2-hydroxy-1-naphthaldehyde. Other data showed that coronary resistance in presence of the naphthalene derivative at a dose of 0.001 nM was higher (p = 0.05) in comparison with 4-nitrobenzoyl azide, 2-hydroxy-1-naphthaldehyde [0.001 nM] and control conditions (Figure 5).
 |
Figure 4: Effect induced by the naphthalene derivative on perfusion pressure. The results show that the naphthalene derivative significantly increase perfusion pressure (p = 0.05) through time in comparison with 4-nitrobenzoyl azide, 2-hydroxy-1-naphthaldehyde and the control conditions. Each bar represents the mean ± S.E. of 9 experiments. |
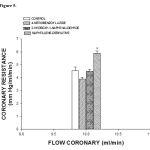 |
Figure 5: Activity exerted by the naphthalene derivative on coronary resistance. The results show that coronary resistance was higher (p = 0.05) in the presence of the naphthalene derivative in comparison with 4-nitrobenzoyl azide, 2-hydroxy-1-naphthaldehyde and the control conditions. Each bar represents the mean ± S.E. of 9 experiments. |
Third stage
Other results showed that activity exerted by the naphthalene derivative increases the left ventricular pressure at dose of 0.001 to 100 nM and this effect was not inhibited by prazosin, propanolol, metoprolol (Figure 6) or indomethacin (Figure 7) at dose of 1 nM.
![Figure 6. Activity exerted by the naphthalene derivative on LVP through of adrenergic receptors. Naphthalene derivative [0.001 to 100 nM] was administered (intracoronary boluses, 50 µl) and the corresponding effect on the LVP was evaluated in the absence and presence of prazosin, propranolol or metoprolol at a dose of 1 nM. The results showed that activity induced by the naphthalene derivative on LVP was not inhibited in the presence of prazosin, propanolol or metoprolol. Each bar represents the mean ± S.E. of 9 experiments. LVP = left ventricular pressure.](https://biomedpharmajournal.org/wp-content/uploads/2015/11/Vol8_No1_New_Marc_fig6-150x150.jpg) |
Figure 6: Activity exerted by the naphthalene derivative on LVP through of adrenergic receptors. Naphthalene derivative [0.001 to 100 nM] was administered (intracoronary boluses, 50 µl) and the corresponding effect on the LVP was evaluated in the absence and presence of prazosin, propranolol or metoprolol at a dose of 1 nM. The results showed that activity induced by the naphthalene derivative on LVP was not inhibited in the presence of prazosin, propanolol or metoprolol. Each bar represents the mean ± S.E. of 9 experiments. LVP = left ventricular pressure. |
Finally, other results (Figure 8) showed that the naphthalene derivative at dose of 0.001 nM and this effect was significantly inhibited in presence of nifedipine (1 nM).
![Figure 7. Effects induced by the naphthalene derivative on LVP through prostaglandins synthesis. Intracoronary boluses (50 µl) of the naphthalene derivative [0.001 to 100 nM] were administered and the corresponding effect on the LVP was determined. The results showed that naphthalene derivative increase the LVP in a dependent dose manner and this effect was not inhibited in the presence of indomethacin [1 nM]. Each bar represents the mean ± S.E. of 9 experiments. LVP = left ventricular pressure.](https://biomedpharmajournal.org/wp-content/uploads/2015/11/Vol8_No1_New_Marc_fig7-150x150.jpg) |
Figure 7: Effects induced by the naphthalene derivative on LVP through prostaglandins synthesis. Intracoronary boluses (50 µl) of the naphthalene derivative [0.001 to 100 nM] were administered and the corresponding effect on the LVP was determined. The results showed that naphthalene derivative increase the LVP in a dependent dose manner and this effect was not inhibited in the presence of indomethacin [1 nM]. Each bar represents the mean ± S.E. of 9 experiments. LVP = left ventricular pressure. |
Fourth stage
Other results (Table 3) showed that the naphthalene derivative increase the levels of intracellular calcium as a consequence of increases in the time; nevertheless, this effect is significantly reduced by nifedipine (1 nM).
Table 3: Activity induced by the naphtalene derivative [0.001 nM] on intracellular calcium levels n absence or presence of nifedipione [1 nM].
|
Cai2+ [mmol] ± SD —————————————————————————————————————————————————————— Time (min) N-D N-D + Nifedipine |
||
|
3 6 9 12 15 18
|
1.30 × 10-3 ± 0.34 1.30 × 10-3 ± 1.02 1.30 × 10-3 ± 0.88 2.00 × 10-3 ± 0.76 2.00 × 10-3 ± 1.00 2.00 × 10-3 ± 0.78 |
7.00 × 10-4 ± 1.04 7.00 × 10-4 ± 0.98 7.00 × 10-4 ± 0.78 7.00 × 10-4 ± 0.74 7.00 × 10-4 ± 0.88 7.00 × 10-4 ± 0.76 |
Discussion
Synthesis chemical
There are several procedures for the synthesis of naphtalen-derivatives [20-22]; nevertheless, expensive reagents and special conditions are required; therefore, in this study a new naphthalene-derivative was synthesized by the reaction of p-nitrobenzoyl azide with 2-hydroxy-1-naphthaldehyde in acid medium (Figure 1). The structure of the naphthalene-derivative was confirmed using IR and NMR spectroscopy (Table 1 and 2). The IR spectra contained characteristic vibrations at 3400 for hydroxyl group; at 1240 for nitro group; at 1670 for amide group. The 1H NMR spectrum of the naphthalene derivative shows signals at 7.00-7.70, 7.90, 8.20-8.62 ppm for naphthalene group; at 7.82 and 8.00 for phenyl group; at 11.62 for hydroxyl group. The 13C NMR spectra displays chemical shifts at 17.70-123.40, 125.28-128.58, 131.44-138.38 and 155.48 ppm for naphthalene group; at 124.48, 130.04-130.18 and 142.02-152.64 ppm for phenyl group; at 175.08 ppm for amide group; at 179.00 for carbon bound to both hydroxyl and amide groups. Finally, the presence of the naphthalene derivative was further confirmed from mass spectrum which showed a molecular ion at m/z 336.06.
![Figure 8. Effects induced by the naphthalene derivative on LVP through calcium channel activation. Intracoronary boluses (50 µl) of the naphthalene derivative [0.001 to 100 nM] were administered and the corresponding effect on the LVP was determined. The results showed that the naphthalene derivative increase the LVP in a dependent dose manner and this effect was significantly inhibited (p = 0.05) in the presence of nifedipine. Each bar represents the mean ± S.E. of 9 experiments. LVP = left ventricular pressure.](https://biomedpharmajournal.org/wp-content/uploads/2015/11/Vol8_No1_New_Marc_fig8-150x150.jpg) |
Figure 8: Effects induced by the naphthalene derivative on LVP through calcium channel activation. Intracoronary boluses (50 µl) of the naphthalene derivative [0.001 to 100 nM] were administered and the corresponding effect on the LVP was determined. The results showed that the naphthalene derivative increase the LVP in a dependent dose manner and this effect was significantly inhibited (p = 0.05) in the presence of nifedipine. Each bar represents the mean ± S.E. of 9 experiments. LVP = left ventricular pressure. |
Biological activity
There are several reports which indicate that some drugs have been used to treat the infarction/reperfusion injury resulting from myocardial ischemia [23-25]; nevertheless, there is scarce information about the effects induced by some naphthalene derivatives involved this phenomenon. Therefore, in this study the activity of a naphthalene derivative on the infarction/reperfusion injury was evaluated using several strategies.
First stage
In order to evaluate the activity of the naphthalene derivative on injury by ischaemia/reperfusion an isolated heart model was used. The results showed that the naphthalene derivative reduced infarct size. This phenomenon induced by the naphthalene derivative can be by activation of some structure biological (p.e. ionic channels or specific receptors) involved in the endothelium of coronary artery [26] or by the influence exerted by the naphthalene derivative on blood pressure which could result reduction in the infarct size, and decrease the myocardial injury after ischemia-reperfusion; it is important to mention that this effect was similar to other reports for other type of drugs [27].
In order to evaluate this hypothesis, the activity induced by the naphthalene derivative on blood vessel capacity and coronary resistance; translated as changes in perfusion pressure was evaluated in an isolated rat heart model. The results showed that the naphthalene derivative significantly increase the perfusion pressure over time. Analyzing this data and evaluating the possibility that functional groups involved in the chemical structure of naphthalene derivative could be by itself the responsible of their activity on perfusion pressure or coronary resistance; in this study, the compounds 4-nitrobenzoyl azide and 2-hydroxy-1-naphthaldehyde were used such pharmacological tool. The results showed that perfusion pressure was not affected in presence of 4-nitrobenzoyl azide and 2-hydroxy-1-naphthaldehyde; therefore, these data confirm that functional groups different involved in the naphthalene derivative are the responsible of the activity exerted on perfusion pressure, which could result in changes in coronary resistance. To evaluate this hypothesis, the effects induced by the naphthalene derivative, 4-nitrobenzoyl azide and 2-hydroxy-1-naphthaldehyde on coronary resistance were evaluated. The results showed that coronary resistance was higher in presence of the naphthalene derivative in comparison with these compounds.
On the other hand, in the search of molecular mechanism of activity induced by the naphthalene derivative on perfusion pressure, in this study was analyzed a report, which indicates that some naphthalene-derivatives can induce changes on blood pressure via adrenergic system [7]. To evaluate these hypotheses, the activity exerted by the naphthalene-derivative on left ventricular pressure in the absence or presence of prazosin, propanolol or metoprolol was evaluated. The results showed that naphthalene derivative increases the left ventricular pressure in a manner dose-dependent and this effect was not inhibited by prazosin, propanolol or metoprolol. All these data indicate that the molecular mechanism involved in the effects of this compound on left ventricular pressure was not through adrenergic activity. In addition, also were analyzing other reports which indicate that some drugs may induce its effect on left ventricular pressure via prostaglandins synthesis [29]; therefore, in this study the activity exerted by the naphthalene derivative on left ventricular pressure in the absence or presence of indomethacin was examined to evaluate the possibility that the activities exerted by the naphthalene derivative involve stimulation and secretion of prostaglandins. The results showed that the naphthalene derivative increased the left ventricular pressure and this effect was not blocked in presence of indomethacin; these data indicate that activity exerted by the naphthalene derivative on left ventricular pressure was not via prostanoids synthesis.
Analyzing these data and other studies which suggest that some steroid derivatives can exert their activity on left ventricular pressure through calcium channels activation [29]; in this study, the effect exerted by the naphthalene derivative on left ventricular pressure was evaluated using nifedipine as pharmacological tool to characterize whether this mechanism was involved in their pharmacological activity. The results showed that the naphthalene derivative increases the left ventricular pressure in a dose dependent manner; however, this effect was blocked in presence of nifedipine. These data suggest that activity exerted by the naphthalene derivative was via calcium channel type L activation. Nevertheless, to evaluate this hypotheses and analyzing other reports which suggested that the effect exerted by some drugs on left ventricular pressure involving increase in intracellular calcium [30]. In this study the effect induced by the naphthalene derivative on intracellular calcium concentration trough of time was evaluated in absence or presence of nifedipine. The results showed that the naphthalene derivative increase the levels of intracellular calcium as a consequence of increases in the time; nevertheless, this effect is significantly reduced by nifedipine. Therefore, all these data indicate that activity exerted by the naphthalene derivative was via calcium channels type L which brings consequently an increasing in the calcium intracellular concentration.
Conclusions
The naphthalene derivative is a particularly interesting drug, because the activity induced for this compound on injury by ischemia/reperfusion involves a molecular mechanism different in comparison with other drugs. This phenomenon may constitute a novel therapy for ischemia/reperfusion injury.
Conflicts of interest
The authors declare that they have no conflict of interest.
References
- Yusuf S, Hawken S, Ounpuu S. Obesity and the risk of myocardial infarction in 27 000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640-9.
- Thygesen K, Alpert J, White H. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50:2173-95.
- Pfeffer M. Left ventricular remodeling after acute myocardial infarction. Annu Rev Med. 1995;46:455-66.
- Klone R, Przyklener K, Whittaker P. Deterious effects of oxygen radicals in ischemia/reperfusion. Circulation. 1989;80: 1115-27.
- Cole W, McPherson C, Sontag D. ATP-regulated K+ channels protect the myocardium against ischemia/reperfusion damage. Circ Res. 1991;69:571-581.
- Griffiths E, Halestrap A. Protection by Cyclosporin A of Ischemia/Reperfusion-Induced Damage in Isolated Rat Hearts. J Mol Cel Cardiol. 1993;25:1461-9.
- Rossoni R, Manfredi B, Cavalca V, Razzetti R, Bongrani S, Polvani G. The Aminotetraline Derivative (±)-(R,S)-5,6-Dihydroxy-2-methylamino-1,2,3,4-tetrahydro-naphthalene Hydrochloride (CHF-1024) Displays Cardioprotection in Postischemic Ventricular Dysfunction of the Rat Heart. J Pharmacol Exp Ther. 2003;307:633-9.
- Sugawara E, Nakayama Y, Senoo Y, Teramoto S. Protective effects of calmodulin antagonists (trifluoperazine and W-7) on hypothermic ischemic rat hearts. Acta Med Okayama. 1991;45:129-34.
- Tian-Lun Y, Mei-Fang C, Jun-Ling J, Qi-Ying X, Yun-Ping L. The endothelin receptor antagonist decreases ischemia/reperfusion-induced tumor necrosis factor production in isolated rat hearts. Inter J Cardiol. 2005;100:495-8.
- Westaby D, Bihari D, Gimson D, Crossley I, Williams R. Selective and non-selective beta receptor blockade in the reduction of portal pressure in patients with cirrhosis and portal hypertension. Gut. 1984;25:121-4.
- Graham R, Oates H, Stoker L, Stokes G, Alpha blocking action of the antihypertensive agent, prazosin. J Pharmacol Exper Ther. 1977;201:747-52.
- Bengtsson C, Johnsson G, Regardh C. Plasma levels and effects of metoprolol on blood pressure and heart rate in hypertensive patients after an acute dose and between two doses during long-term treatment. Clin Pharmacol Ther. 1975;17:400-8.
- Owen T, Ehrhart I, Weidner W, Scott J, Haddy F. Effects of indomethacin on local blood flow regulation in canine heart and kidney. Exp Biol Med. 1975;149:871-6.
- Henry P. Comparative pharmacology of calcium antagonists: nifedipine, verapamil and diltiazem. Am J Cardiol. 1980;46:1047-58.
- Bayne K. Revised guide for the care and use of laboratory animals available. Am Physiol Soc. 1996;39:208-11.
- Figueroa-Valverde L, Díaz-Cedillo F, López-Ramos M. Actividad inotrópica inducida por el derivado carbamacepina-alquino en un modelo de corazón aislado y perfundido a flujo constante. Biomedica. 2011;31:232-41.
- Booth E, Obeid N, Lucchesi B. Activation of estrogen receptor-α protects the in vivo rabbit heart from ischemia-reperfusion injury. AJP-Heart. 2005;289:H2039-47.
- Boe J, Khan B. Colorimetric determination of blood calcium. J Biol Chem. 1929;81:1-8.
- Hocht C, Opezzo L, Gorzalczany S. Una aproximación cinética y dinámica de metildopa en ratas con coartación aórtica mediante microdiálisis. Rev Argent Cardiol. 1999;67:769-73.
- Weber G, Farris F. Synthesis and spectral properties of a hydrophobic fluorescent probe: 6-propionyl-2-(dimethylamino)naphthalene. Biochem. 1979;18:3075-8.
- House H, Koepsell D, Campbell W. Synthesis of some diphenyl and triphenyl derivatives of anthracene and naphthalene. J Org Chem. 1972;37:1003-11.
- Yoshikawa E, Yoshinori Y. Palladium-Catalyzed Intermolecular Controlled Insertion of Benzyne-Benzyne-Alkene and Benzyne-Alkyne-Alkene-Synthesis of Phenanthrene and Naphthalene Derivatives. Ang Chem Inter. 2000;39:173-5.
- Node K, Kitakaze M, Kosaka H. Amelioration of Ischemia- and Reperfusion-Induced Myocardial Injury by 17β-Estradiol. Circulation. 1997;96:1953-63.
- Suparto I, Koudy W, Fox J. A comparison of two progestins on myocardial ischemia–reperfusion injury in ovariectomized monkeys receiving estrogen therapy. Coronary Artery Dis. 2005;16:301-8.
- Jeanes H, Wanikiat P, Sharif I. A Medroxyprogesterone acetate inhibits the cardioprotective effect of estrogen in experimental ischemia-reperfusion injury. Menopause. 2006;13:80-6.
- Bouïs D, Hospers G, Meijer C. Endothelium in vitro: A review of human vascular endothelial cell lines for blood vessel-related research. Angiogenesis. 2000;4:91-102.
- Beer S, Reincke M, Kral M. Susceptibility to cardiac ischemia/reperfusion injury is modulated by chronic estrogen status. J Cardiov Pharmacol. 2002;40:420-8.
- Sheillan C, Ody C, Russo F, Duval D. Differential aspects of sex steroids on prostaglandin secretion by male and female cultured piglet endothelial cells. Prostaglandins. 1983;26:3-12.
- Figueroa-Valverde L, Díaz-Cedillo F, López-Ramos M. Design and synthesis of an estradiol derivative and evaluation of its inotropic activity in isolated rat heart. African J Pharm Pharmacol. 2011;5:1703-12.
- Figueroa-Valverde L, Díaz-Cedillo F, García-Cervera E, Pool-Gómez E, López-Ramos M, Rosas-Nexticapa M. Positive inotropic activity induced by a dehydroisoandrosterone derivative in isolated rat heart model. Arch Pharm Res. 2013;36:1270-8.







