Hashemi Seyede Sara1, khaksar Zabiholah2, Rafati Ali Reza3
1Assistant professor, Shiraz Burn Research Centre, Shiraz University of Medical Sciences, Shiraz, Iran. 2Professor of Comparative Anatomy and Embryology, Department of Anatomy, Faculty of Veterinary, Shiraz University, Shiraz, Iran. 3Department of Pharmacology, Islamic Azad University, Sarvestan Branch, Sarvestan, Iran. Corresponding author E-mail sara_hashemi@sums.ac.ir
DOI : https://dx.doi.org/10.13005/bpj/636
Abstract
This study investigated the effect of Juglans regia leaves ethanolic extract of maternal diabetes on fetal brain structure, especially in cerebrum. A total of 24 Sprague-Dawley female rats became diabetic by intraperitoneal injection of streptozotocin (50 mg/kg). The animals were divided randomly into four groups. Rats in all groups became pregnant by natural mating. At day 1, 14 and 28 days after birth, the cerebrum was collected from offspring of all rats and neonatal weight was measured. Various histological parameters were determined using histological techniques. Results revealed a significant decrease in number of cells in gray matter and white matter at 1, 14 and 28 days after birth and a significant decrease in thickness of gray matter and molecular layer at day 14 post neonatal in cerebrum in offspring of diabetic mothers as compared with other groups. Maternal diabetes has significant deleterious effects on cerebrum and leads to decrease in the number of neurons and change in the shape of brain. Walnut leaf extract prevents harmful effects of diabetes on the brain structure in offspring of diabetic mothers.
Keywords
Juglans regia leaves extract; Cerebrum; Maternal diabetes; Offspring; Rat
Download this article as:| Copy the following to cite this article: Sara H. S, Zabiholah K, Reza R. A. Morphometric Study of the effect of Walnut (Juglans regia) leaf extract on Cerebrum malformation in offsprings of diabetic rats. Biomed Pharmacol J 2015;8(1) |
| Copy the following to cite this URL: Sara H. S, Zabiholah K, Reza R. A. Morphometric Study of the effect of Walnut (Juglans regia) leaf extract on Cerebrum malformation in offsprings of diabetic rats. Biomed Pharmacol J 2015;8(1). Available from: http://biomedpharmajournal.org/?p=941 |
Introduction
Diabetes mellitus is a group of metabolic alterations characterized by hyperglycemia resulting from defects in insulin secretion, action, or both. It has already been established that chronic hyperglycemia resulting from diabetes is associated with long term damage, dysfunction, and eventually the failure of organs, especially the eyes, kidneys, nerves, heart and blood vessels (Huang et al., 2005). In diabetic mothers during pregnancy, placental transport of glucose and other nutrients will be increased due to an increased availability at the maternal site, resulting in their increase in fetal and neonatal Macrosomia (Persson and Hanson, 1998). The risk for diabetes is significantly higher in the offspring of mothers who have non-insulin-dependent diabetes (Knowler et al., 1985). In addition, maternal diabetes increases the risk of hypoglycaemia and other chemical imbalances like low calcium and magnesium levels (Jones, 2001). One of the mammalian systems that is clearly impaired in diabetes is nervous system. Diabetes leads to decreased sensation at the nerve endings (Cecil et al. 2003). Atherosclerosis in brain is one of the prominent changes in diabetes. Studies have shown that obstruction of feeding vessels of nerves due to diabetes causes nerve bundles death and myelin destruction (Harrison et al., 2000). An increased number of malformations occur in infants born from mothers with maternal diabetes involving the central nervous system (CNS), the spinal column, the ribs and the urinary tract (Aberg, 2002).
Herbal drugs are gaining popularity in the treatment of diabetic mellitus (Pari and Uma., 1999). The Juglans genus (family Juglandaceae) comprises several species and is widely distributed throughout the world (Anonimous, 1999). Green walnuts, shells, kernels and seeds, bark and leaves have been used in the pharmaceutical and cosmetic industries (Stampar et al., 2006). Walnut leaves are considered a source of healthcare compounds, and have been intensively used in traditional medicine for treatment of venous insufficiency and haemorrhoidal symptomatology, and for its antidiarrheic, antihelmintic, depurative and astringent properties (Van Hellemont, 1986; Bruneton, 1993; Wichtl and Anton, 1999). Keratolytic, antifungal, hypoglycaemic, hypotensive, anti-scrofulous and sedative activities have also been described (Valnet, 1992; Gıˆrzu et al., 1998). Walnut (Juglans regia) has been widely used as an herbal medicine in the treatment for diabetes (Asgary et al., 2008).
In walnut leaves, naphtoquinones and flavonoids are considered as major phenolic compounds (Wichtl and Anton, 1999). Juglone (5-hydroxy-1,4-naphthoquinone) is known as being the characteristic compound of Juglans spp. and is reported to occur in fresh walnut leaves (Bruneton, 1993; Wichtl and Anton, 1999; Gıˆrzu et al., 1998; Solar et al., 2006). Nevertheless, because of polymerization phenomena, juglone only occurs in dry leaves in vestigial amounts (Wichtl and Anton, 1999).
The purpose of this investigation is the effect of Walnut (Juglans regia) leaf extract to evaluate possibility of congenital cerebral malformation in offspring of diabetic rats at day 14 and 28 after birth.
Materials and Methods
Ethanolic Extract of Juglans regia Leaves (JRLEE)
Leaves of Juglans regia L. (Juglandaceae) were collected from Fars province. The leaves were cleaned, shed dried at 25°C and ground with a blender. The powdered leaves of J. regia (1000g) were then allowed to soak for about 24 h in 70% ethanol and re-extracted three times with fresh 96% ethanol at room temperature for 24 h. The ethanolic phases were pooled and the residue was removed by filtration. The ethanolic extract was concentrated at 40°C by rotary evaporator and then lyophilized to obtain a powder (JRLME). The powder was stored in the dark at 4°C for subsequent experiments.
Animals
Twenty four adult female Sprague-Dawley rats (200-250g weight and 3-4 months old) were acclimatized in an environmentally controlled room (temperature, 22±2oC, and 12h light/12h dark). Food and water were given ad libitum. In this study all experiments conducted on animals were in accordance with the guidance of the Ethical Committee for Research on Laboratory Animals of Shiraz University. Animals were divided into four equal groups.
Induction of diabetes mellitus
Adult rats were rendered hyperglycemic by a single intraperitoneal (I.P.) injection of B.W. of stereptozocin (Sigma Chemical Co., USA) (50 mg/kg body weight) (Szkudelski, 2001). Diabetes was identified by polydipsia, polyuria and by measuring non–fasting serum glucose concentration 48h after the injection of STZ, rats with a blood glucose level over 250 mg/dl were considered to be diabetic.
Experimental design
Animals were divided into four identical groups as follows:
- Normoglycemic control group (NC): normal rats which received distilled water
- Normoglycemic treated group (NJRLE): normal rats which received the Walnut leaf extract (JRLE, 250 mg/kg B.W)
- Diabetic control group (DC): Diabetic rats treated with distilled water.
- Diabetic treated group (DJRLE): diabetic rats receiving the Walnut leaf extract (JRLE, 250 mg/kg B.W)
Female animals of four groups in oestrus stage were caged with male rat for mating. Mating was confirmed by vaginal plug observation (Turner and Bagnara 1971). Each group included 6 rats and animals were given the extract orally by an intragastric tube once daily for 21 days. The stock solution was prepared for multiple groups, such that 1 mL of extraction was administered per day for each animal.
After 1, 14 and 28 days of birth, 8 infants were selected randomly from each groups and the body weight were measured.Then the animals were euthanized and their cerebrum removed. The cerebrum samples were immersed in 5% buffered formalin to be prepared for optical microscope.
Histomorphometric study
All tissue samples were fixed in 5% buffered formalin fixative for histolopathological investigations and subsequently embedded in paraffin. Sections (5 microns thickness) were stained with H and E and Green Masson’s trichrome techniques. Sections were observed with Olympus BX51 microscope for evaluation of histomorphometrical parameters such as:
1) Thickness of gray matter (μm), 2) Thickness of white matter (μm), 3) Thickness of molecular layer, 4) The number of cells in the gray matter per unit, 5) The number of cells in white matter per unit, 6) The ratio of gray matter to white matter.
Thicknesses of gray matter, white matter and molecular layer were measured by ocular micrometer and Olympus BX51 light microscope using Olysia software. The number of cells per unit in both white and gray matters and the ratio of gray matter to white matter were counted by ocular graticule and Olympus BX51 light microscope using Olysia software.
Statistical analysis
All values were expressed as mean ± standard deviation (SD). Significant differences among the groups were determined by one way analysis of variance (ANOVA) followed by Duncan’s test to analyze the difference using the Statistical Package for Social Sciences (SPSS) 16.0 software package programs. Values of P ≤ 0.05 were taken as statistically significant.
Results
The body weight changes in offspring of diabetic rats after 1, 14 and 28 days of birth in the four groups is shown in Table 1. Mean body weight in the neonatal of diabetic mothers was significantly (P<0.05) more than that of the other groups.
Table 1: Comparison of means and standard error of the body weight in offsprings of diabetic rats at 1, 14 and 28 days after birth.
| Group | NC | DC | NJRLE | DJRLE |
| 1 | 5.15±0.33 | 6.55±0.38* | 5.22±0.31 | 5.55±0.31 |
| 14 | 18.64±1.94 | 27.42±1.38* | 18.88±1.22 | 20.28±1.64 |
| 28 | 61.31±4.91 | 74.71±4.43* | 61.12±3.17 | 61.35±4.21 |
Table 2 shows different parameters of offspring’s cerebrum of four groups, at 1 day after the birth. The thickness of gray matter was decreased significantly (P<0.05) in 1-day old newborns of diabetic rats compared to that of the other groups. The number of cells in gray matter and white matter was significantly (P<0.05) decreased in offsprings of diabetic rats compared to that of the other groups. The thickness of white matter was decreased in diabetic rat fetuses compared to the other groups and this reduction was not significant, while ratio of gray matter to white matter in offsprings of diabetic rats was significantly (P<0.05) decreased compared to the other groups.
Table 2: Comparison of means and standard error of the cells number and dimension Cerebrum at 1 day after birth.
| Days | 1 | 1 | ||
| Group | NC | DC | NJRLE | DJRLE |
| TGM(μ) | 503.12±35.83 | 415.68±32.04* | 491.71±33.88 | 478.28±26.94 |
| TWM(μ) | 322.34±18.39 | 334.18±25.25 | 319.51±20.29 | 321.39±17.86 |
| NGM(n/mm2) | 26742.62±781.61 | 23138.52±654.12* | 26514.86±702.45 | 25413.32±658.32 |
| NWM(n/mm2) | 11221.88±401.32 | 9986.71±387.83* | 11125.14±368.76 | 10985.72±391.54 |
| GWR | 1.66±0.09 | 1.32±0.03* | 1.71±0.11 | 1.57±0.08 |
Table2: TGM (Thickness of gray matter), TWM (Thickness of white matter), NGM (Number of cells in gray matter), NWM (Number of cells in white matter), GWR (Ratio of gray matter to white matter), Values are demonstrated with mean± SD. Significant difference between DC and other groups demonstrated with*sign (P<0.05).
Table 3 shows various cerebrum parameters of 14 days old -offsprings of four groups.
Table 3: Comparison of the means and standard error of the cells Number and dimension Cerebrum at 14 day after birth
| Days | 14 | 14 | ||
| Group | NC | DC | NJRLE | DJRLE |
| TGM(μ) | 686..21±51.51 | 561.36±38.78* | 682.92±48.55 | 611.46±49.91 |
| TWM(μ) | 521.42±32.17 | 467.99±31.79 | 516.33±36.58 | 486.94±37.98 |
| TML(μ) | 148.15±13.09 | 118.02±11.78* | 146.13±12.47 | 138.12±12.97 |
| NGM(n/mm2) | 4152.53±251.01 | 3271.14±194.15* | 4121.33±237.48 | 3941.15±116.61 |
| NWM(n/mm2) | 2714.44±169.39 | 2111.81±194.15* | 2721.13±124.11 | 2681.11±146.29 |
| GWR | 1.21±0.09 | 1.11±0.09 | 1.19±0.05 | 1.14±0.06 |
Table 3: TGM (Thickness of gray matter), TWM (Thickness of white matter), TML(Thickness of molecular layer), NGM (Number of cells in gray matter), NWM (Number of cells in white matter), GWR (Ratio of gray matter to white matter), Values are demonstrated with mean± SD. significant difference between DC and other groups demonstrated with*sign ( P<0.05).
Table 4 shows various cerebrum parameters of 28 days old-offsprings of four groups. The thickness of gray matter and molecular layer in offsprings of diabetic mother at 14 and 28 days of post-neonatal period was decreased significantly (P<0.05) compared to that of the other groups.Table 3 shows various cerebrum parameters of 14 days old -offsprings of four groups.
Table 4: Comparison of the means and standard error of the cells Number and dimension Cerebrum at 28 day after birth.
| Days | 28 | 28 | ||
| Group | NC | DC | NJRLE | DJRLE |
| TGM(μ) | 555.86±47.91 | 524.87±41.03 | 553.47±46.75 | 538.12±43.63 |
| TWM(μ) | 569.34±13.05 | 544.18±23.14 | 563.87±29.65 | 558.99±27.95 |
| TML(μ) | 198.19±17.19 | 148.02±15.78 | 193.26±16.37 | 162.75±13.94 |
| NGM(n/mm2) | 3241.12±255.62 | 2524.61±271.97* | 3236.41±281.04 | 3111.54±252.49 |
| NWM(n/mm2) | 2051.81±186.71 | 1612.81±153.77* | 2032.61±173.15 | 1963.12±174.26 |
| GWR | 0.92±0.1 | 0.91±0.07 | 0.93±0.06 | 0.92±0.08 |
Table 4: TGM (Thickness of gray matter), TWM(Thickness of white matter), TML(Thickness of molecular layer), NGM (Number of cells in gray matter), NWM (Number of cells in white matter), GWR (Ratio of gray matter to white matter), Values are demonstrated with mean± SD. significant difference between DC and other groups demonstrated with*sign ( P<0.05).
The thickness of white matter in offsprings of diabetic mother was decreased compared to that of the other groups and this reduction was not significant. The ratio of gray matter to white matter in 14 and 28 days old-offsprings of diabetic mother was decreased compared to that of the other groups, and the reduction was not significant.
Figures 1 and 2 demonstrate the comparison of the cerebrum cell numbers of four groups at 14 days after birth.
Figure 1: Photo Micrograph part of the outer grainy layer of cerebral cortex in neonatal rat 14 days after birth in normal control (NC)(A) DC(B) NJRLE(C) and DJRLE(D) groups.Picture shows a reduction of the number of neurons in group B than in group A, C and D (Staining: Hematoxilin-Eosin).
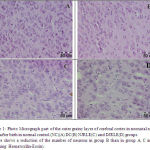 |
Figure 1 |
Figure 2: Photo Micrograph part of the inner pyramidal layer of cerebral cortex in neonatal rat 14 days after birth in normal control (NC)(A) DC(B) NJRLE(C) and DJRLE(D) groups.Picture shows a reduction of the number of neurons in group B than in group A, C and D (Staining: Hematoxilin-Eosin).
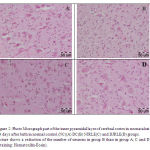 |
Figure 2 |
Discussion
In the present study, it is shown that Juglans regia Leaves Ethanolic Extract (JRLME) decreases the blood glucose levels. Streptozotocin (STZ) is widely used to induce experimental diabetes in animals (Szkudelski, 2001). STZ action in β cells is accompanied by characteristic alterations in blood insulin and glucose concentrations. STZ impairs glucose oxidation (Bedoya et al., 1996) and decreases insulin biosynthesis and secretion (Bolaffi et al., 1987; Nukatsuka et al., 1990b). It was observed that STZ at first abolished the B cell response to glucose. Temporary return of responsiveness then appears, which is followed by its permanent loss and cells are damaged (West et al., 1996).
The body weight in offspring of diabetic mothers was significantly more than that of
other groups (Macrosomia), which is due to increase in placental transport of glucose and other nutrients (Jones, 2001). In a previous study, the body weight of offspring of diabetic rats was increased significantly (Khaksar et al., 2010).
The thickness of gray matter in cerebrum was significantly decreased in offspring of diabetic mother as compared to that of other groups at day 14 postneonatal, whereas the thickness of white matter in this region was insignificantly decreased in offspring of diabetic mother as compared to that of other groups. Therefore, maternal diabetes results in malformation of this region. Neuropathy of numerous nerves like sciatic nerve has been reported in offspring of diabetic mother (Artico et al., 2002; Guyton and Hall, 2006). Malformations in this region of brain may occur due to neuropathy. Diabetes mellitus is associated with moderate cognitive deficits and neurophysiological and structural changes in the brain, a condition that may be referred to as diabetic encephalopathy. The emerging view is that the diabetic brain features many symptoms that are best described as “accelerated brain ageing” (Biessels et al., 2002). Maternal diabetes leads to white matter hyperintensities and gray matter density changes in fetus (Musen et al. 2006). Maternal diabetes leads to fetal hyperbilirubinemia (Cuningham et al., 2005), that could result in an encephalopathy which is named Kernicterus (Murry et al., 2003).
Table 3 and 4 demonstrate a decrease in the number of cells in gray matter and white matter in offspring of diabetic mother as compared to that of other groups. In diabetic pregnancy, maternal glucose transport to fetal blood via the placenta (Jones, 2001) and increase in fetal blood glucose may result in diabetic neuropathy in fetus, as diabetes leads to neuropathy in adult (Guyton and Hall, 2006). Hyperglycemic condition disturbs the proliferation and cell death of neural progenitors in mouse embryonic spinal cord (Gao and Gao, 2007). Hyperglycemia effectively makes more substrate available for aerobic glycolysis in the brain, leading to acidosis (Biessels et al., 1994) and enhanced oxygen free radical formation by reduction in levels of protective endogenous antioxidants (Baydas et al., 2002). These radicals contribute to increased neuronal death by oxidizing proteins, damaging DNA, and inducing the lipoperoxidation of cellular membranes (Hawkins and Davies, 2001). Diabetes may enhance the development of stroke via increased cortical apoptotic activity, but this was not additive in the hippocampus following ischemic injury (Li et al., 2004). Maternal diabetes induces some changes in hippocampus neuronal structure and density. Statistical analysis show significant decrease in neuronal density (ND) in neonates from diabetic mothers compared to control (Tehranipoor and Khakzad, 2008). In a previous study, the mean number of cells in gray matter and white matter increased in diabetic rats fetus that were fed walnut leaf extract compared to diabetic rats fetus, indicating the extract has a beneficial effect in the treatment of maternal diabetes(Hashemi et al., 2015).
The hypoglycemic activity of walnut leaves was reported in previous studies (Fukuda et al., 2004) Walnut leaves constitute a good source of antioxidant compounds, namely phenolics, suggesting that it could be useful in prevention of diseases in which free radicals are implicated. Phenolic acid and flavonoid are two major groups of phenolic compounds in walnut leaves. The most important phenolic acid in walnut leaf is caffeoylquinic acid and the main flavonoid is quercetin (Fukuda, 2003; Pereira et al., 2007; Solar et al., 2006). Some studies have shown that flavonoids are able to decrease plasma glucose level (Li et al., 2004). Quercetin inhibits glucose transporter (GLUT2), and so diminishes glucose intestinal absorption (Kwon, 2007). Caffeoylquinic acid (chlorogenic acid) is a specific inhibitor of glucose-6-phosphate translocase and reduces hepatic glucose production (Hemmerle, 1997), thus decreasing blood glucose level and HbA1C (Dhandapani, 2002). Plant antioxidants are able to restore and regenerate pancreatic B cells. The results from the studies on garlic, onion and fenugreek show that in diabetic rats treated with antioxidants, the number of Langerhans islets has increased significantly (El–demerdash, 2005). Walnuts also contain several phytosterols, as it appears that they can inhibit intestinal absorption of cholesterol (Fukuda et al. 2004) demonstrated a strong inhibitory activity of walnut polyphenols and the polyphenolic components like Casuarictin, tellimagradin II and Tellimagradin I on different enzymes like glycosidase, sucrose, maltase and amylase. In addition to the above findings, researchers also noticed that walnut polyphenol-rich fraction has triglyceride lowering effect and urine peroxide lowering effect in genetically inherited Type II diabetes mellitus (db/db) mice at the dose of 200mg/kg/day. The consumption of walnut leaf pellets in alloxan induced diabetic rats at the dose of 185 mg/kg, reduced fasting blood sugar significantly and the histomorphometric study of pancreas showed a sign of regeneration of β-cells in the treated group (Jelodar et al., 2007). J. regia leaves methanolic extract at dose of 250 mg/kg decreases the postprandinal plasma blood glucose levels in both short and long term models. The plant extract significantly inhibited α-glucosidase activity in vitro for both maltase and sucrase enzymes and showed no changes in the insulin and glut-4 genes expression. The author attributed the inhibitory action of the plant extract to gallic acid and caffeoylquinic acid in the leaves (Teimori et al., 2010).
In conclusion, maternal diabetes has significant deleterious effects on cerebrum and leads to decrease in the number of neurons and change in the shape of brain. Walnut leaf extract prevents harmful effects of diabetes on the brain structure in offspring of diabetic mothers.
References
- Aberg A, Westbom L, kallen B. (2002) congenital malformation among infants whose mothers had gestational diabetes or pre-existing diabetes. Early Human Development 61:85-95
- Anonimous, (1999). Recenseamento Geral Agrı´cola. Instituto Nacional de Estatı´stica, Portugal
- Artico M, Massa R, Cavallotti D, Franchitto S, Cavallotti, C (2002) Morphological changes in the sciatic Nerve of Diabetic Rats Treated with low Molecular weight Harparin op 2123/parnaparin. Anat Histol Embryol 31:193-197
- Asgary S, Parkhideh S, Solhpour A, Madani H, Mahzouni P, Rahimi P . (2008) Effect of ethanolic extract of Juglans regia L. on blood sugar in diabetes-induced rats. Med. Food 11(3): 533-538.
- Baydas G, Canatan H, Turkoglu A (2002) Comparative analysis of the protective effects of melatonin and vitamin E on streptozocin-induced diabetes mellitus. J Pineal Res. 32:225-230
- Bedoya FJ, Solano F, Lucas M (1996). N-monomethyl-arginine and nicotinamide prevent streptozotocin-induced double strand DNA break formation in pancreatic rat islets. Experientia 52: 344-347.
- Biessels GJ, Kappelle AC, Bravenboer B, Erkelens DW, Gispen WH (1994) Cerebral function in diabetes mellitus. Diabetologia 37:643-650
- Biessels GJ, Vander Heide LP, Kamal A, Bleys RL, Gispen WH (2002) Ageing and diabetes: implications for brain function. European journal of pharmacology 441:1-14
- Bolaffi JL, Nagamatsu S, Harris J, Grodsky GM (1987). Protection by thymidine, an inhibitor of polyadenosine diphosphate ribosylation, of streptozotocin inhibition of insulin secretion. Endocrinology 120: 2117-2122.
- Bruneton, J, (1993). Pharmacogosie, phytochimie, plantes me´dicinales and Doc.- Lavoisier, Paris, p. 348.
- Cecil RF, Goldman L, Ausiello DA (2003).Cecil Textbook of medicine. (22nd ed), W.B. Saunders co ,Philadelphia, pp 1095-1104
- Cuningham FG, Lolo KG, Blome AL, Hat JC (2005) William’s obstetrics. (22nd ed). New York, McGraw-Hill, pp1170-1187
- Dhandapani S, Subramanian VR, Rajagopal S, Namasivayam N (2002).Hypolipidemic effect of Cuminum cyminum L. on alloxan– induced diabetic rats. Res. 46: 251-255.
- El–demerdash FM, Yousef M I, Abou El – Naga NI (2005). Biochemical study on the hypoglycemic effects of onion and garlic in alloxan induced diabetic rats. Food Chem. Toxicol 43: 57-63.
- Fathiazad F, Garjani A, Motavallian naini A.( 2006). Study of hypoglycemic activity of the hydroalcoholic extract of Juglans regia in normal and diabetic rats. Pharm. Sci 2: 13 – 7.
- Fukuda T, Ito H and Yoshida T. (2004). Effect of the walnut polyphenol fraction on oxidative stress in type 2 diabetes mice. Biofactors 21, pp. 251-253.
- Gao Q. and Gao YM. (2007). Hyperglycemic condition disturbs the proliferation and cell death of neural progenitors in mouse embryonic spinal cord. J. Neurosci. 25, No.6, pp.349-357.
- Gıˆrzu M., Carnat A, Privat A M, Fiaplip J, Carnat A P, Lamaison J L. (1998). Sedative effect of walnut leaf extract and juglone, an isolated constituent Pharmaceutical Biology 36, 280–286.
- Guyton AC, Hall JE. (2006) Textbook of medical physiology. (11th ed). Elsevier Saunders, Philadelphia, pp 961-976
- Harrison TR, Braunwal DE, Wilson JD. (2000) Harrison’s principles of internal medicine. McGraw Hill, New York, pp 2109-2142
- Hashemi SS, Khaksar Z, and Tadjalli M.(2015). Morphometric study of the effect of Walnut (Juglans regia) leaf extract on cerebrum malformation in fetuses of diabetic rats. Journal of Chemical and Pharmaceutical Research 7(2):441-445
- Hemmerle H, Burger HJ, Below P, Schubert G, Ripple R, Schindler PW, Paulus E, Herling AW (1997). Chlorogenic acid and synthetic chlorogenic acid derivatives: novel inhibitors of hepatic glucose-6- phosphate translocase. Med. Chem. 40: 137-145.
- Hawkins CL. and Davies MJ. (2001). “Generation and propagation of radical reactions on proteins.” Biophys. Acta1. 504, pp.196-219.
- Huang THW, Peng G, Kota BP, Li GQ, Yamahara J, Roufogalis BD, Li Y.(2005). Anti-diabetic action of Punica granatum fl ower extract: activation of PPAR-c and identifi cation of an active component. Toxicol Appl Pharmacol 207: 160-169.
- Jelodar, GhA, Maleki M, and Sirus Sh. (2007). Effect of walnut leaf, coriander and pomegranate on blood glucose and histopathology of pancreas of Alloxan induced diabetic rats. J. Trad. CAM 4 (3): 299 – 305
- Jones CW. (2001) Gestational diabetes and its impact on the neonate. Neonatal Network 20(6):17-23
- Knowler W, Pettitt DJ, Kunzelman CL, Everhart J. (1985) Genetic and environment determinants of non-insulin dependent diabetes mellitus. Diabetes Res Clin Practice 1:309
- Kwon O, Esk P, Chen S, Corpe CP, Lee JH, Kruhlak M, Levine M. (2007) Inhibition of the intestinal glucose transporter GLUT2 by flavonoids. FASEB J. 21: 366-377.
- Li ZG, Britton M, Sima AA, Dunbar JC. (2004) Diabetes enhances apoptosis induced by cerebral ischemia. Life Sci 76:249-262
- Nukatsuka M, Yoshimura Y, Nishida M, Kawada J. (1990b) Importance of the concentration of ATP in rat. pancreatic beta cells in the mechanism of streptozotocin-induced cytotoxicity. J. Endocrinol 127: 161-165
- Moordian AD. (1997) Central nervous system complication of diabetes mellitus- a perspective from the blood brain barrier. Brain Res Rev 23(3):210-218
- Murry RK, Graner DK, Mayes PA, Rodwell VW. (2003) Harper’s Illustrated Biochemistry. McGraw Hill, New York, pp 270-285
- Musen G, Lyoo IK, Sparks CR. (2006) Effects of Type 1 Diabetes on Gray Matter Density as Measured by Voxel-Based Morphometry. Diabetes 1(55):326-333
- Pari L, Uma MJ. (1999) Hypoglycemic eff ect of Musa sapientum L. in alloxan-induced diabetic rats. J Ethnopharmacol. 68: 321-325.
- Pereira JA, Oliveira I, Sousa A, Valentao P, Andrade P, Ferreira I, Ferreres F, Bento A, Seabra R, Estevinho L. (2007). Walnut (Juglans regia L.) leaves: phenolic compound, antibacterial activity and antioxidant potential of different cultivars. Food Chem. Toxicol 45(11): 2287-2295.
- Persson B, Hanson U (1998) Neonatal morbidities in gestational diabetes mellitus. Diabetes Care 2:79-84
- Solar A, Colaric M, Usenik V, Stampar F. (2006) Seasonal variation of selected flavonoids, phenolic acids and quinones in annual shorts of common walnut (Juglans regia L.). Plant Science 170, 461–543.
- Stampar, F., Solar, A., Hudina, M., Veberic, R., Colaric, M.,( 2006). Traditional walnut liqueur – cocktail of phenolics. Food Chemistry 95, 627–631.
- SzkuDelski T (2001) The mechanism of Alloxan and Streptozotocin action in B Cells of the rat pancreas. physiol Res 50:536-546
- Tehranipour M, Khakzad MR (2008) Effect of Maternal Diabetes on Hippocampus Neuronal Density in Neonatal Rats. Journal of Biological Sciences 8(6):1027-1032
- Teimori shaban M, Montaser kohsari Sh, Ghafarzadegan R, Haji rezai R.(2010) Antidiabetic Effects of Juglans regia Leave’s Methanolic Extract . on alloxan-induced diabetic rats. J Biomed Phar 2 (34): 142 –9.
- Turner CD, Bagnara JT. (1971) General endocrinology. WB Saunders co, Philadelphia, pp 516-522
- Van Assche FA, Aerts L, De prins FA. (1983) Degranulation of the insulin-producing B cells in an infant of a diabetic mother. British Journal of obstetrics and Gynaecology 90:182-185
- Valnet J. (1992). Phytothe´rapie Traitement des maladies par les plantes. Maloine, Paris, pp. 476–478.
- Van Hellemont (1986). Compendium de phytotherapie. Association Pharmaceutique Belge, Bruxelles, pp. 214–216.
- West E, Simon OR, Morrison EY. (1996). Streptozotocin alters pancreatic beta-cell responsiveness to glucose within six hours of injection into rats. West Indian Med. J., 45: 60-62.
- Wichtl M., Anton R., (1999). Plantes the´rapeutiques. and Doc., Paris, pp. 291–293.







