Janardhan Raj and Kiran Babu Uppuluri*
Bioprospecting Laboratory, Department of Biotechnology, School of Chemical and Biotechnology, SASTRA University, Thanjavur-613401, Tamil Nadu, India. Corresponding Author Email: kinnubio@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/585
Abstract
Nano particle formulations, especially protein based formulations are great drug delivery systems for a targeted and sustained drug release. The Specificity lies with the receptor-ligand binding mechanism adopted by them. Metformin hydrochloride (MET) is a widely used biguanide oral antidiabetic agent, against Type II diabetes. In its free form, it suffers some shortcomings like poor oral bioavailability for its site specific absorption and frequent dosage administration requirement for its short half life. With a vision of troubleshooting these drawbacks, we developed a novel protein based, ‘casein-MET micelles’ delivery system. It is a sustained drug delivery system that involves a very simple and reliable method of using sodium caseinate for entrapping MET. These synthetic micelles were characterized by their particle size distribution, zeta potential, polydispersity index. The morphological and physicochemical characterization was done by using Scanning electron microscopy and FTIR spectroscopy, which was further validated by in-vitro drug release evaluation. This method produced very fine and stable casein-MET micelles with a uniform rod shape of average 1.2µm size. In-vitro drug release behavior of casein micelles suggests that about half of the drug is released during first 3h and 85% of the drug is released after 15h of its administration, which could be a great improvement of the existing methods.
Keywords
Metformin (MET); sodium caseinate; micelles; In-vitro release
Download this article as:| Copy the following to cite this article: Raj J, Uppuluri K. B. Metformin Loaded Casein Micelles for Sustained Delivery: Formulation, Characterization and in-Vitro Evaluation. Biomed Pharmacol J 2015;8(1) |
| Copy the following to cite this URL: Raj J, Uppuluri K. B. Metformin Loaded Casein Micelles for Sustained Delivery: Formulation, Characterization and in-Vitro Evaluation. Biomed Pharmacol J 2015;8(1). Available from: http://biomedpharmajournal.org/?p=989 |
Introduction
Nanotechnology plays a prominent role in the development of pharmaceuticals and biomedical agents especially for the effective drug delivery at the site of action [1]. Biopolymers have been widely used for this purpose, due to their biocompatibility, biodegradability, non-toxicity, and non-antigenicity. Proteins are recognized as suitable candidates for encapsulating the drug at nano/micro scale as they offer excellent absorbability and low toxicity. In addition, the protein encapsulated drug possesses active targeting ability, enhanced permeation and retention effect (EPR effect) [2]. In this context, proteins such as albumin, casein, gelatin and silk protein based nano formulations including- films [3, 4], hydrogels [5], micro-particles [5] and nanoparticles [6] have been developed as the drug delivery systems.
Nanotechnology derived formulations are based on molecular self-assembly and co-assembly of polymers. The molecules of a major milk protein, casein which have a natural self-assembling tendency, co-assemble and organize themselves to form spherical micelles of 50–1000 nm size. Casein is a proline rich protein containing distinct hydrophobic and hydrophilic domains. In recent years, there is a considerable interest in developing casein based controlled drug delivery systems for their cost-effectiveness, easy availability, non-toxicity and higher biodegradability[7]. Also the physicochemical properties like casein’s affinity of attachment to smaller molecules; its surface-active and stabilizing properties; its emulsification, gelation and water binding capacities; it offers an interesting and promising protein for encapsulation of both hydrophobic and hydrophilic drugs for sustained drug release. Further caseins’ shielding capabilities ensure protection, bio-accessibility and enhanced bioavailability of sensitive drugs that are being encapsulated [4]. Number of casein encapsulated nano and micro formulations are reported in the recent years [8, 9] [10].
Diabetes is a chronic metabolic disorder and one of the major causes of death and disability in the world. By 2025 diabetes may affect as many as 300 million people worldwide and most of the cases will be Type II diabetes [11]. Type II diabetes is the most common type of diabetes and also called as non-insulin-dependent diabetes as it can be evidenced by insulin resistance, during which the body produces normal or excess levels of insulin but fails to utilize it, leading to hyperglycemia in the individual [12].
Metformin (MET) is an oral antidiabetic agent that belongs to the biguanide class and is considered as a first line of drug for Type II diabetes. It increases insulin sensitivity and glucose tolerance by lowering both basal and postprandial glucose levels. MET lowers the intestinal absorption of glucose and hepatic gluconeogenesis. It enhances glycogenesis, lipogenesis, glucose uptake by adipocytes and muscle cells [13, 14]. In addition MET activates protein kinase which plays a major role in insulin signaling thus maintaining body’s energy balance with proper metabolism of glucose and fats. Interestingly, MET is the only oral antidiabetic which provides cardiovascular protection besides its property of reduction of blood glucose levels [15, 16].
MET is BCS (Biopharmaceutical Classification System) class III drug and has been available for its clinical use over decades. MET has its own absorption window in the GI tract, hence shows poor oral bioavailability (50-60%). It also needs frequent doses of administration to elicit therapeutic response i.e., 500 mg dose two or three times a day for its short half life (1.5-4.5h) [15]. One of its few rare side effects called “lactic acidosis”, causes mortality in 50 % of patients associated with MET therapy, besides chest pain, diarrhea, abdominal discomfort, vomiting, stomachache, headache are common [17]. Keeping in view of its clinical benefits, novel methods were developed to overcome its drawbacks and improved formulations of MET were prepared, especially of sustained release drug delivery system kind and were reported during the recent past e.g., Extended release tablets [18], Non-ionic surfactant vesicles [19], Matrix tablets [20], Controlled release floating drug delivery system [21, 22], Sustained release matrix tablets [20] , Extended-release matrix tablets [23], Niosomes [24] and Nano formulations for MET etc. were also reported. Among them, protein encapsulated [25] MET nano formulations showed efficient results.
The main aim of this research was to study the entrapment phenomenon of a first line type II antidiabetic drug, MET in casein micelles, and there by the assessment of its sustained release pattern from the entrapped micelle. The morphological and physico- chemical characterization of the casein loaded MET micelles was done. The interaction between casein and MET was studied by using FTIR spectroscopy. Particle size, polydispersity index (PDl) and zeta potential of micro particles were evaluated by Photon Correlation Spectroscopy (PCS). Scanning electron microscopy (SEM) was used to study the morphological characteristics of microspheres. The entrapment efficiency of MET in micro spheres was assessed and the in-vitro release profile was evaluated.
Materials and methods
Chemicals
Metformin hydrochloride (MET) and sodium caseinate were purchased from Sigma Aldrich, India. All the other chemicals and reagents of analytical grade were obtained from Merck India.
Metformin entrapment into casein
Briefly, 2mL of 1%MET solution was added to 20mL of 5%sodium caseinate in phosphate buffer with continuous stirring. The samples were equilibrated for 16h at room temperature (30-35oC) [26]. Each experiment was performed in triplicates and mean ± SE values were recorded.
Characterization of MET loaded casein micelles
The size and the zeta potential of MET loaded casein micelles were measured by Photon Correlation Spectroscopy (PCS) using a Malvern Zeta sizer 3000 HS with He-Ne laser (633 nm) at a scattering angle of 90o. The interaction between casein and MET was studied by using FTIR spectroscopy using potassium bromide (KBr) pellet technique in which, samples were ground in a mortar with KBr (1:100) and pressed in a hydraulic press (9 tons) to small tablets which were then analyzed by transmittance technique with 4 cm-1 resolution. The spectra of free sodium caseinate, pure MET, MET in alkaline, caseinate-MET suspension and MET encapsulated casein micelles were recorded on a Perkin Elmer FTIR spectrometer (Perkin Elmer, India) in a range of about 4000-400cm-1range. The morphology and size characteristics of the MET encapsulated casein micelles were studied by Scanning electron microscopy (SEM) (JEOL JSM 670F-6701) at a voltage of around 3 keV.
Spectroscopic determination of Metformin
The determination of MET in solution was done by visible Spectroscopy according to Hassan et al 1999 [27]. Aliquots (0 – 1.0mL) of 0.2mg/mL MET solutions were prepared, to which 2.5mL of 0.2 M copper (II) sulfate, 12.5mL of cyclohexylamine were added and was made up to 25mL of final volume with distilled water. The mixture was thoroughly mixed for 15 min and then filtered. The absorbance of the resultant colored reaction mixtures was measured at 545nm using Thermo Evolution 201 UV visible spectrophotometer. Calibration graph was plotted, regression equation was fitted and correlation coefficient value (R) was calculated.
Entrapment efficiency of Metformin
Metformin entrapped casein micelles were centrifuged at 16000 rpm for 1 h and the supernatant was collected. The amount of casein micelles entrapped MET was measured by visible spectrophotometer at 545 nm. Measurements were recorded in triplicate and the mean value ± standard error was reported.
In- vitro drug Release studies
Lyophilized casein micelles were accurately weighed and dispersed to the original volume in phosphate buffer at pH 7.4. Release pattern of MET from CASEIN-MET micelles was observed by using Dissolution apparatus USP type 1 (Basket). It consists of a cellophane tube tied with a dialysis membrane that was kept in a basket type apparatus filled with PBS (pH 7.4). The CASEIN-MET micelles were placed inside the dialysis membrane and drug release studies were carried out at room temperature. The samples were withdrawn periodically and measured for recording respective MET concentrations at specific time periods. The volume of dissolution medium was kept constant by the addition of PBS for every sample collection. The same procedure was adopted for both drug and formulation for a period of about 18h. The experiments were carried out in triplicate and results were reported as the mean ± standard error.
Results and Discussion
Sustained release formulation of Metformin looks promising in reducing the side effects and enhancing the patient’s compliance with the drug. In recent years, hydroxyl propyl methylcellulose (HPMC) controlled matrices of MET were developed. Even though these extended matrix tablets showed good results, still there is a lot of scope for further development of fast, effective and reliable formulations for effective delivery of MET at their specific sites of action. Therefore in this present work, the formulation and evaluation of CASEIN entrapped MET micelles had been demonstrated.
Preparation and characterization of CASEIN MET micelles
The preparation of solid matrix tables is a tedious process, hence a simple method was chosen to formulate MET into a sustained release formulation with biopolymer casein. The CASEIN-MET micelles were prepared by aforementioned method. The average hydrodynamic size of MET-loaded CASEIN micelles was 963.4 nm (Fig. 1A). The poly dispersity index (pdi) was found to be 0.669, indicating the presence of different sized particles and a similar trend was observed in SEM micrographs. The particle size and surface charge are important parameters of MET entrapped micelles for the body’s defense mechanism in their recognition or ignorance. Therefore, we measured the zeta potential of the CASEIN-MET NPs (Fig. 1B). Zeta potential value obtained for CASEIN-MET micelles was -23.7, and the negative sign indicates the alkaline nature of the micelles in the pH scale. The stability of the colloidal system is mostly dependent on the negative charge of the particles and there by the preventing the columbic repulsive forces between the particles that cause their agglomeration in the colloidal state [28, 29]. A minimum zeta potential value of – 20 mV is highly desirable in case of combined electrostatic and stearic stabilization of nanoparticles [30].
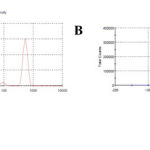 |
Figure 1: (A) Particle size distribution (inset, appearance of CASEIN-MET micelles) and (B) zeta potential of CASEIN-MET micelles. |
FTIR spectra of pure MET, MET in alkaline, CASEIN and CASEIN-MET and CASEIN-MET micelles are shown in Fig. 2. No chemical interaction of MET and sodium caseinate was observed from the FTIR spectra. In Metformin spectrum, biguanide C=N stretching vibrations were seen at 1647, 1580 and 1550cm-1 and also the C-N stretching occurred in the region of 1300cm-1wave number. Whereas N-H asymmetric and symmetric vibrations were observed in the region of 3400-3500 cm-1range. The frequent C=O stretching frequencies that were associated with the sodium caseinate spectrum could be due to its secondary structural elements. The suspension and subsequent CASEIN-MET micelles exhibited similar pattern of C=O, C=N, C-N stretching and vibrations in the range of 1200-3400 cm-1wave number. The strong absorption peak of secondary amides at 3550cm-1 might infer the encapsulation of MET within protein. Similar peak pattern was observed in spectra of IFTR spectroscopy from the literature [31].
 |
Figure 2: FTIR spectra of bulk MET, MET in alkaline medium, sodium caseinate, CASEIN-MET suspension and CASEIN-MET micelles |
SEM of CASEIN-MET micellec is shown in Fig. 3, which appeared to be rod shaped with smooth surfaces and of nearly uniform size. The average size of the micelles indicated by SEM was about 1.2µm.
Entrapment efficiency of Metformin
The amount of encapsulated drug could be determined by its entrapment efficiency in micelles in relation to the total amount of drug used [32]. At the chosen experimental condition, the encapsulation efficiency of MET within CASEIN micelles was calculated to be 87.42 ±0.91%. The % leakage of MET could be due to colaescence and disruption of emulsion droplets and drug diffusion from aqueous phase to oil phase which was investigated upon storage at 4oC after 2, 4 and 8 weeks. Entrapment efficiency was found more in matrix tablets prepared with the HPMC polymers, i.e., almost 90-95 % efficiency ( found in literature) with all solid matrix tablets [31]. Even though the entrapment efficiency is a little low in the present work, the proposed method was very simple and convenient for formulation of MET for sustained and slow drug release pattern.
 |
Figure 3: Scanning electron micrograph of CASEIN-MET micelles. |
In- vitro drug Release studies
The dissolution tests for both MET and CASEIN-MET micelles were studied and the bulk MET dissolution rate was compared with that of CASEIN-MET micelles and the final results were produced in Fig 4. From the figure, a rapid release of MET during the initial stages was observed in both pure and nano formulations. This could be due to the desorption and diffusion of MET from the outer surface of micelles. It is always preferred to have initial burst from the controlled drug delivery systems to attain a threshold dose for proper elicitation of therapeutic action. After the initial rapid release, CASEIN-MET micelles showed a constant slow and sustained release and the maximum drug was released in 15h, whereas the pure drug showed the initial burst release and was shown the maximum amount of drug release within 3h of its administration. The slow release pattern of micelles after initial rapid release had been reported earlier and our system was consistent with literature and showed slow diffusion of MET from their micelles [19, 25].
fig4
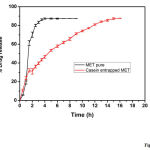 |
Figure 4: In vitro drug release profile for bulk MET and CASEIN-MET micelles. |
Extended release tablets also showed a slow and sustained release with the maximum drug release at 10 hrs [31]. Only two nano formulations of MET were reported, i.e., MET loaded niosomes [19] and BSA entrapped MET [25] for sustained drug release and thereby enhancing patients’ compliance with MET.
In order to maintain safer therapeutic concentrations of a drug for a stipulated time period, always a slow and sustained drug release formulation is desirable to avoid any toxic systemic effects. Hence, we suggest that the CASEIN-MET micelles offer an effective controlled drug delivery system with reduced systemic side effects and enhanced targeting efficiency.
Conclusion
The method of adoption of Protein nanoparticles as drug delivery devices has been emerged as a promising and efficient form of formulating various classes of drugs, which offers a greater biocompatibility, biodegradability and sustained drug release when compared to other existing methods. In the present work, CASEIN-MET micelles were successfully formulated by a very simple, fast and reliable method. The FTIR spectroscopic studies were performed for drug, protein, nano formulation, drug- protein samples; whose IFTR spectral analyses depicted proper entrapment of drug in the protein micelles and no chemical interaction between MET and casein. In-vitro release profiles of free drug and micelles suspensions confirmed that this colloidal drug carrier was capable of releasing drug in a well controlled manner. The formed micelles showed good stability every time, in spite of the repetitive execution of the same method for a test period of six months, which obviated the validity of the method adopted. However, this formulation method requires in-vivo testing for a complete assurance of its stability and safety prior to its commercial scale up.
References
- Salouti, M. and A. Ahangari, Nanoparticle based Drug Delivery Systems for Treatment of Infectious Diseases. 2014.p.155-192.
- Zhen, X., et al., Cellular uptake, antitumor response and tumor penetration of cisplatin-loaded milk protein nanoparticles. Biomaterials, 2013. 34(4): p. 1372-1382.
- Chen, L., et al., Kinetics of the breakdown of cross-linked soy protein films for drug delivery. Biomaterials, 2008. 29(27): p. 3750-3756.
- Ma, J., et al., Synthesis and biological response of casein-based silica nano-composite film for drug delivery system. Colloids and Surfaces B: Biointerfaces, 2013. 111: p. 257-263.
- Kuijpers, A., et al., In vitro and in vivo evaluation of gelatin–chondroitin sulphate hydrogels for controlled release of antibacterial proteins. Biomaterials, 2000. 21(17): p. 1763-1772.
- Jun, J.Y., et al., Preparation of size-controlled bovine serum albumin (BSA) nanoparticles by a modified desolvation method. Food Chemistry, 2011. 127(4): p. 1892-1898.
- Livney, Y.D., Milk proteins as vehicles for bioactives. Current Opinion in Colloid & Interface Science, 2010. 15(1): p. 73-83.
- Elzoghby, A.O., W.S.A. El-Fotoh, and N.A. Elgindy, Casein-based formulations as promising controlled release drug delivery systems. Journal of controlled release, 2011. 153(3): p. 206-216.
- Elzoghby, A.O., W.M. Samy, and N.A. Elgindy, Novel spray-dried genipin-crosslinked casein nanoparticles for prolonged release of alfuzosin hydrochloride. Pharmaceutical research, 2013. 30(2): p. 512-522.
- SAHLAN, M. and T. SUPARDI, Encapsulation of indonesian propolis by Casein micelle. International Journal of Pharma and Bio Sciences, 2013. 1(4).
- Zimmet, P., Diabetes epidemiology as a tool to trigger diabetes research and care. Diabetologia, 1999. 42(5): p. 499-518.
- Mellitus, D., Diagnosis and classification of diabetes mellitus. Diabetes care, 2005. 28: p. S37.
- Viollet, B., et al., Cellular and molecular mechanisms of metformin: an overview. Clinical science, 2012. 122(6): p. 253-270.
- Stepensky, D., et al., Preclinical evaluation of pharmacokinetic–pharmacodynamic rationale for oral CR metformin formulation. Journal of controlled release, 2001. 71(1): p. 107-115.
- Gusler, G. and B. Berner. Metformin (GR) gastric retentive tablets: GI transit and pharmacokinetics in healthy volunteers. in Millennial World Congress of Pharmaceutical Science, San Francisco, California. 2000.
- El Messaoudi, S., et al., The cardioprotective effects of metformin. Current opinion in lipidology, 2011. 22(6): p. 445-453.
- Rojas, L. and M.B. Gomes, Metformin: an old but still the best treatment for type 2 diabetes. Diabetol Metab Syndr, 2013. 5(1): p. 6.
- Timmins, P., et al., Steady-state pharmacokinetics of a novel extended-release metformin formulation. Clinical pharmacokinetics, 2005. 44(7): p. 721-729.
- Sankhyan, A. and P.K. Pawar, Metformin loaded non-ionic surfactant vesicles: optimization of formulation, effect of process variables and characterization. Daru, 2013. 21(1): p. 7.
- Mandal, U., et al., Formulation and optimization of sustained release matrix tablet of metformin HCl 500 mg using response surface methodology. JOURNAL-PHARMACEUTICAL SOCIETY OF JAPAN, 2007. 127(8): p. 1281.
- Patel, A., S. Ray, and R.S. Thakur, Invitro evaluation and optimization of controlled release floating drug delivery system of metformin hydrochloride. DARU Journal of Pharmaceutical Sciences, 2006. 14(2): p. 57-64.
- Couvreur, P., et al., Nanotechnologies for drug delivery: Application to cancer and autoimmune diseases. Progress in solid state chemistry, 2006. 34(2): p. 231-235.
- Nanjwade, B.K., S.R. Mhase, and F. Manvi, Formulation of extended-release metformin hydrochloride matrix tablets. Tropical journal of pharmaceutical research, 2011. 10(4): p. 375-383.
- Hasan, A.A., H. Madkor, and S. Wageh, Formulation and evaluation of metformin hydrochloride-loaded niosomes as controlled release drug delivery system. Drug delivery, 2013. 20(3-4): p. 120-126.
- Jose, P., et al., Metformin-loaded BSA nanoparticles in cancer therapy: a new perspective for an old antidiabetic drug. Cell biochemistry and biophysics, 2014: p. 1-10.
- Semo, E., et al., Casein micelle as a natural nano-capsular vehicle for nutraceuticals. Food Hydrocolloids, 2007. 21(5): p. 936-942.
- Hassan, S.S., et al., Determination of metformin in pharmaceutical preparations using potentiometry, spectrofluorimetry and UV–visible spectrophotometry. Analytica chimica acta, 1999. 378(1): p. 299-311.
- Wilson, B., et al., Albumin nanoparticles for the delivery of gabapentin: Preparation, characterization and pharmacodynamic studies. International journal of pharmaceutics, 2014. 473(1): p. 73-79.
- Mainardes, R.M. and R.C. Evangelista, PLGA nanoparticles containing praziquantel: effect of formulation variables on size distribution. International journal of pharmaceutics, 2005. 290(1): p. 137-144.
- Kohane, D.S., et al., Biodegradable polymeric microspheres and nanospheres for drug delivery in the peritoneum. Journal of Biomedical Materials Research Part A, 2006. 77(2): p. 351-361.
- !!! INVALID CITATION !!!
- Silva, R., et al., Protein microspheres as suitable devices for piroxicam release. Colloids and Surfaces B: Biointerfaces, 2012. 92: p. 277-285.








