Manuscript accepted on :
Published online on: 10-11-2015
Plagiarism Check: Yes
Arshadi Abotaleb1, Kargar Jahromi Hossein2*, Farokhnia Farzaneh3, Rahmanian Elham4, Shafiei Jahromi Nazanin5, Farzam Mohammad6
1Master of Physiology, Darab Department of Education, Fars Province, Iran. 2Zoonoses Research Center, Jahrom University of Medical Sciences, Jahrom, Iran. 3Master of Physiology, Shiraz Department of Education, Fars Province, Iran. 4Anatomy and embryology, Cellular and Molecular Gerash research center, Grash school of medical science, Shiraz University of medical Science, Shiraz, Iran. 5Department of Nursing, Islamic Azad University, Firuzabad Branch, Firuzabad, Iran. 6Department of Anatomy and Embryology, Shiraz University of medical Science, Shiraz, Iran. Corresponding Author: hossein.kargarjahromy@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/608
Abstract
Introduction: Cardiovascular diseases are now considered as the first cause of death in industrialized countries. Over the past few decades in most countries, the use of alternative treatments and especially herbal therapy and dietary supplements are increased by people to improve a variety of diseases such as high blood cholesterol. So the main goal of the study is investigating on protective effects of barley and oat seeds extracts on some blood parameters (HDL, LDL, and glucose) in female rats fed with a high-fat diet. Methods:in the study, a number of 50 female Wistar rats weighing approximately 20± 300 g, were bought from Jahrom’s animals house. Then they were divided into 5 groups with 10 rats per each, including control group (no material received), blank group (received 1% cholesterol by weight of consumed food), experimental 1 (daily received 1% cholesterol by weight of consumed food, and 125 mg/kg.BW dose of barley extract), experimental 2 (daily received 1% cholesterol by weight of consumed food, as well as 125 mg/kg.BW dose of oat extract), experimental 3 (daily received 1% cholesterol by weight of consumed food, as well as 62.5 mg/kg.BW dose of oat extract). At the end of the experiment animals were bled and HDL, LDL and glucose factors were evaluated. Results: pathological examinations of blood factors showed that LDL level in the experimental group that simultaneously received barley and oat had significant reduction than the group that received cholesterol alone. Also LDL level had significant increase in the experimental group receiving oats only compared to the group receiving barely and oat simultaneously (P<05/0). HDL concentration showed also a significant decrease in all the experimental groups and the amount of glucose showed significant reduction in the experimental groups receiving barley + oat, and barley and oat only in compared to the control group (P<05/0). Conclusions: given to the results can state that barley and oat extracts reduce the risk of cardiovascular diseases due to having vitamins and effective ingredients. The simultaneous use of barley and oat extracts is recommended.
Keywords
Barley; Oat; Pathological Changes; Blood Factors; Rat
Download this article as:| Copy the following to cite this article: Abotaleb A, Hossein K. J, Farzaneh F, Elham R, Nazanin S. J, Mohammad F. Investigating on Protective Effects of Barley and Oat Seeds Extracts on Pathological Changes of Blood Factors in Female Rats Fed with High-Fat Diet. Biomed. Pharmacol. J.;8(1) |
| Copy the following to cite this URL: Abotaleb A, Hossein K. J, Farzaneh F, Elham R, Nazanin S. J, Mohammad F. Investigating on Protective Effects of Barley and Oat Seeds Extracts on Pathological Changes of Blood Factors in Female Rats Fed with High-Fat Diet. Biomed Pharmacol J 2015;8(1). Available from: http://biomedpharmajournal.org/?p=542 |
Introduction
Now cardiovascular diseases are considered as the first cause of death in industrialized countries. Reports indicate that the disease caused death of 950 thousand people in the United States in 1998 and the country has sustained about 118 billion dollars for the disease in 2000 (1). Increase of blood lipids especially cholesterol proposes as a significant factor in intensification of these diseases (2). Over the past few decades, the amount of using alternative therapies especially herbal therapy and dietary supplements have been increased by people in most countries to improve variety of diseases such as high blood lipids. One of the major problems facing doctors and also consumers of medicinal plants is lack of sufficient information about drugs’ safety and their impacts on diseases. Fortunately, during the past 30 years extensive researches have been done on the effectiveness of medicinal plants used in traditional medicine that proves the efficiency or inefficiency of them (3).
Most of barley species especially agricultural barleys which are planted in different parts of the world are from Sativum species (4). According to Vaviouf theory the origin and location of primary distribution of barley have been in Syria (H. Spontaneum) and Tibet in central Asia (H. Agriocrithon) includes awn barley with short awn and naked seeds (4). Hordium genus includes two species. This group has large seeds barleys that are entirely one-year old and have 2-14n chromosomes and include: (H. stivium) H.vulgare, their main axis is fragile and includes two- and six-row types and they are cultivable (4). H. agriocrithon is a kind of barley and obtained from central Asia regions, it has fragile main axis, spikes with awn, naked seeds and include two and six-rows and is cultivable (4).
Barley seeds with low glycemic index (5, 6) and high fiber content (6), water soluble fibers (7) such as β-glucan and high content of chromium and magnesium (5) are suitable grains for preventing and treating diabetes and blood fat changes (8, 9, 10). Researches that were performed on barley seeds expressed that the plant has anti-diabetic effects. It was also stated that the use of barley malt extract and consumption of a diet containing barley cause reduction of glucose in diabetic animals and also consumption of barley seeds reduces glucose in diabetic people (9, 11). Studies on healthy humans showed that the use of dietary containing barley seeds as well as consumption of β-glucan supplements produced from barley results in reduction of glucose (12).
Among cereals, oat is economically located in the fourth row after wheat, rice and corn and because during the growing period its water requirement is about 500mm, its agriculture is not encouraged in Iran and are cultivated in trace amounts in the North and the West of the country (4).
Oats contain averagely about 7% fats, 5.13 % nitrogen and 60% hydrocarbons. Fatty acids consist of palmitic acid 10%, oleic acid 50 to 60 % and linoleic acid 15 to 30 % (13-14).
Cultivated oats varieties had a modest amount of β-glucan and oil in the past. While we have now modified varieties of oats which contains 18% oil and this amount is twice the amount of oil in old plant varieties, another group of modified varieties of oat has too numerous β-glucan which is called β-glucan (15). Studies have found that in diabetic patients who consuming diet high in oat and other carbohydrates fibers, the amount of consuming insulin will be half in their body. And some who had consumed a lower dose of insulin, completely cut off the drug. As a result, fibers reduce the rate of carbohydrates absorption and prevent a sudden increase of blood glucose (16-18).
Given the importance and influences of barley and oats as well as the importance of prevention and control of cardiovascular diseases, and also since till now no research has been done about barley and oat effects on pathological changes of blood factors including HDL, LDL and glucose, so the main purpose of the present study is to investigate the effect of barley and oat extract on the mentioned parameters and on reduce the risk of cardiovascular disease on hypercholesterolemic rats.
Methods
The study is conducted in vitro and completely random; and all ethical principles for use of laboratory animals have been observed. A total number of 30 adult female Wistar rats weighing 20% ± 300 and 100-120 days of age were obtained from Jahrom’s research center. The rats were kept for 21 days under laboratory conditions including a temperature of 2± 21˚C and a cycle of 12 hours of light and 12 hours of darkness in Jahrom’s animals’ house of Islamic Azad University. The rats were nourished by Pellete and water was provided for them by especial glass bottles. Their cages were disinfected with 70% alcohol 3 times in a week.
To prepare the extract, barley was purchased from market and then was milled well and powdered completely. For preparing aqueous extracts, water distillation system was used. The amount of 100 g of barley and oat seeds was dissolved separately in 1000 ml of distilled water. Then heated in 30-40˚C for two hours and the extract was separated by filtration. The remaining residue on the filter was completely dried in an oven and the amount of dissolved powder was determined. In continues, additional water was concentrated in a rotary device and was kept in dark containers for the next test (19). Preparation method of pressed seeds contain cholesterol is so that Pellete was powdered by a mill. Then 10 kg of powder was weighed and mixed with 100 g of cholesterol. The resulting powder by using of water conversed to a dough-like compound and was brought in the form of plate and then dried in the sun. Each group of animals included 10 female rats. That includes:
Control group: this group had normal diet and received no material
Blank group: this group had a high-fat diet (received 1% cholesterol by weight of consumed food)
Experimental group 1: daily received 1% cholesterol by weight of consumed food, and 125 mg/kg.BW dose of barley extract by intraperitoneally injection.
Experimental group 2: daily received 1% cholesterol by weight of consumed food, as well as 125 mg/kg.BW dose of oat extract by intraperitoneally injection.
Experimental group 3: daily received 1% cholesterol by weight of consumed food, as well as 62.5 mg/kg.BW dose of oat extract by intraperitoneally injection.
After completing a period of 21-day, and weighing all the rats then they became anesthetized by ether and 5ml of blood were taken from their hearts by syringe. After separation of blood serum, pathological concentration of HDL, LDL and glucose factors were measured using enzymatic colorimetric method (PAP-GPO) to determine a single point by photometric method, in a laboratory of University of Medical Science of Jahrom. ANOVA analysis followed by t-test and Duncan test were used for multiple comparisons between different groups. P05/0> was considered as a significant level. The SPSS software version 18 was used for data analysis and performance of statistical tests.
The pathological results of blood factors in the Chart (1) shows that concentration of HDL in the experimental group that received barley and oat has significant increase than the control group and the other experimental groups show significant reduction than the control group (P<0.05).
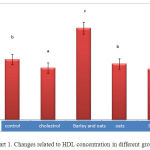 |
Figure 1: Changes related to HDL concentration in different groups |
The amount of LDL in the experimental group that simultaneously received barley and oat shows significant reduction compared to the group that only received cholesterol and the control group (Chart 2). Also the experimental group that only received oat shows a significant increase than the experimental group which simultaneously received barley and oat (P<0.05).
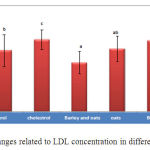 |
Figure 2. Changes related to LDL concentration in different groups |
Pathological glucose level in the experimental groups received barley + oat, barley and oat only shows significant reduction than the control group (P<0.05) (Chart 3).
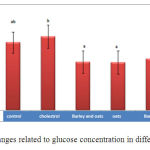 |
Figure 3: Changes related to glucose concentration in different groups |
*The presence of a joint letter does not represent a significant reduction compared to the control group.
Discussion
Deposition of plaques containing cholesterol and fatty materials with fibrosis tissue and calcium salt deposits in intima and/or inner walls of large and medium arteries in various limbs can cause atherosclerosis (20). Atherosclerosis is a complex process that cannot determine a major risk factor for it because different factors and parameters are involved with each other (21). Some important risk factors are including dyslipidemia, glycosylation events, and all events that increase oxidative shock (22-24). It is suggested in the past researches that high level of total cholesterol and LDL has been identified as an important factor for coronary heart disease (25). There is evidence that increase of low-density lipoproteins such as LDL and triglycerides also can cause person to be talented to atherosclerosis (26). A susceptible stage in creation of coronary artery atherosclerosis is oxidation of fatty acids and LDL cholesterol in artery walls. Free radicals produced by metabolism of smooth muscle cells of vascular wall are capable of LDL oxidation (27).
LDL oxidation is a process caused by free radicals which during of it unsaturated fatty acids available in cell membrane become peroxide and production of aldehydes with high reactivity (malondialdehyde) and conjugated dienes increase (28). In this study, the pathological concentration of LDL in the group that received oat increased than the group that simultaneously received barley and oat, indicating the positive effect of simultaneous using of both extracts. Also, the pathological concentration of LDL in the group receiving barley and oat extracts had significant reduction than the group receiving cholesterol. In an review about effects of yeast and β-glucan plant on lipid profile and Secom of probiotic bacteria using rats having high cholesterol diet showed that beta-glucan extract derived from barley lowered more cholesterol and LDL levels and increased HDL level. While beta-glucan derived from yeast less lowers cholesterol so beta-glucan anti-fat effects depend on derived source (29). Researches also suggest that dietary fibers available in oat flour and bran rapidly reduce 20% of blood harmful cholesterol (LDL) level, and increase 15% of good cholesterol (HDL) level (18).
Reports suggested that consumption of vitamins probably by reducing free radicals reduce risk of cardiovascular diseases and in hyperlipidemic patients improve vascular endothelial function (30). Available vitamin C in herbal compounds as an antioxidant reduces the peroxidation of lipids and oxidative degradation of vessels. Using vitamin C and diets rich of antioxidant vitamins also leads to health protection and reduces risk of heart diseases (31). It seems according to the results that vitamin C through two main mechanisms cause favorable changes at HDL and LDL level: By 1. Applying the antioxidant effect that cause reduction of LDL oxidation and increase of its identification by its receptors. 2. Applying competitive effect (due to structural similarity) with glucose in process of HDL and LDL Glycation leads to increase LDL catabolism and reduce HDL excretion (32). What is certain is that oat extract contains different vitamins such as vitamin C that by using the above mechanisms leads to reduction of blood lipids (33, 34).
According to Chart 1, the pathological concentration of HDL in the experimental groups receiving oat and barley alone and also simultaneously decreased and the most effect was related to simultaneous use of extracts. Previous studies have found that high-density lipoprotein has beneficial effects on activity of hyperlipidemic patients and thus reduction of its serum level will follow lipid problems in patients and people with coronary artery disease (35, 36). As mentioned above the high-density lipoprotein level in the experimental group which simultaneously received oat and barley extracts had a significant increase compared to the control group. The increase should be considered useful since this kind of cholesterol causes secretion of harmful cholesterol out of arteries and it is useful to prevent from heart attack and cerebral stroke, so it is better that cholesterol and HDL levels to be high in blood (37). What is certain is that the beneficial effects of extracts are probably due to effective ingredients and soluble vitamins within it that are mentioned before.
Studies found that oat extract contains 10% palmitic acid, 50 to 60% oleic acid and 15 to 30% linleic acid (33, 34) and also found that unsaturated fatty acids reduce pathological LDL which is an atherogenic material (38). Therefore, in this study, the pathological concentration of LDL in the experimental group that simultaneously received the both extracts shows the positive effect of them.
According to the Chart (3) a significant decrease was observed in the groups receiving barley and oat alone and simultaneously. The history of use of barley seeds for feeding and treatment of diseases (39) including diabetes (40, 41) shows that this extract is effective in treatment of diabetes and low blood sugar. It is stated in the past that barley seed is the case that its role in treatment of diabetes was mentioned in ancient texts of Iran’s traditional medicine. In study of Hordeumvulgare barley seed extract effect on concentration of fasting plasma glucose of streptozotocin-induced diabetic rats stated that water extract of barley seed was significantly reduced the level of serum glucose after four weeks in diabetic rats and after a week in non-diabetic rats. In non-diabetic rats the effect was not sustained after the first week. It is clarified in studies that in diabetic patients, who have high-fiber diet of oat and other carbohydrates, level of consumption of insulin receives to half and some people, who take less insulin, cut the drug consumption. Thus, fibers rapidly reduce absorption of carbohydrates and prevent upsurge in glucose (16, 18). So the results of researches are in agreement with the current study. In addition, simultaneous use of barley and oat extracts causes more reduce in glucose level, which is quite logical.
Conclusion
The results showed that barley and oat extracts reduce pathological concentration of LDL and glucose, and increase pathological concentration of HDL with regard to the effective ingredients within the extracts and simultaneous use of both extracts is recommended.
References
- Eisenberg DM, David RB and Ettner SL. Trends in alternative medicine use in the United States, 1990–1997: results of a follow-up national survey. JAMA 1998; 280: 1569–1575.
- Hoerger TJ and Bala MV. Treatment patterns and distribution of low-density lipoprotein cholesterol levels in treatment-eligible United States adults. Am. J. Cardiol. 1998; 82: 61- 65.
- Fallah Hosseini H, Fakhr Zadeh H, Dast Pak A. et al. A review on medicinal plants affecting on high blood lipids. Quarterly Journal of Medicinal Plants; 2003-2004; 4 (11): 9-20.
- Khoda Bandeh N. Cereals. Publication of Tehran University, Seventh Edition, 2002-2001; 1-283.
- Pizzorno Jr JE, Murry MT, Herb Joiner-Bay. The Clinician’s Handbook of Natural Medicine. 1st ed. Churchill Livingstone. China. 2002, pp: 152 – 63.
- Bjorck I, Elmstahl HL. The glycemic index: importance of dietary fibre and other food properties. Proc. Nutr. Soc. 2003; 62 (1): 201 – 6.
- Keogh GF, Cooper GJ, Mulvey TB, McArdle BH, Coles GD, Monro JA, Poppit SD. Randomized controlled crossover study of effect of a highly beta glucan-enriched barley on cardiovascular disease risk factors in mildly hypercholesterolemic men. Am. J. Clin. Nutr. 2003; 78 (4): 711 – 8.
- Jenkins DJ, Wolever TM, Jenkins AL. Starchy foods and glycemic index. Diabetes Care. 1988; 11 (2): 149 – 59.
- Shukla K, Narain JP, Puri P, Gupta A, Bijlani RL, Mahapatra SC, Karmarkar MG. Glycaemic response to maize, bajra and barley. Indian. J. physiol. Phamacol. 1991; 35 (4): 249 – 54.
- Truswell AS. Glycemic index of foods. Eur. J. Clin. Nutr. 1992; 46 (Suppl 2): S91 – 101.
- Routledge. pp. 16–17. ISBN 0-415-02647-4.
- Poppitt SD, van Drunen JD, MacGill AT, Mulvey TB, Leahy FE. Supplementation of a ighcarbohydrate breakfast with barley beta glucan improves postprandial glycaemic response for meals but not beverages. Asia. Pac. J. Clin. Nutr. 2007; 16 (1): 16 – 24.
- Baldridge AD, Perez-Atayde AR, Graeme-Cook F, Higgins L, Lavine JE. Idiopathic steatohepatitis in childhood: a multicenter retrospective study. J Pediatr. 1995;127:700-4.
- Bacon BR, Farahvash MJ, Janney CG, Neuschwander-Tetri BA. Nonalcoholic steatohepatitis: an expanded clinical entity. Gastroenterology. 1994;107:1103-9.
- Molla Zadeh M. scientific and practical production of medicinal plants (planting, harvesting, medicinal properties). Publications of agricultural training and extension. 2012-2013.
- Jarahi M, Zahedi M, Taherian A, Miladi H, Safaghah H. The effect of Matricaria chamomilla L. oil extract on ulcer repair in Rat. J Med Plants 2008; 8(1): 29.
- Zargari A. Herbal plants. Tehran: Tehran University Publisher; 1997.
- Tan PV, Dimo T, Dongo E. Effects of methanol, cyclohexane and methylene chloride extracts of Bidens pilosa on various gastric ulcer models in rats. J Ethnopharmacology 2000; 73: 415-21.
- Hassanzadeh H. 1991. Vegetables grown in the garden and Printing, Bureau of Agricultural Engineering, 174- 175.
- Witztum 2004. Thematic Reviews on Thepathogensis of atherosclerosis. Atherosclerosis, 45: 991- 992.
- Keaneyt 2000. Atherosclerosis: from lesion formation to plaque activation and endothelial dysfunction. Mol Aspects Med , 21: 99-166.
- Clermont P , Creager M , Lorsordo D, Gregory k, Dzau 2005. Atherosclerosis: recent discoveries and novel hypoyheses. Circulation, 112: 3348-3353.
- Katsuyuki N, Takamitsu N, Akira 2006. The oxidative modification hypothesis of atherosclerosis: The comparison of atherogenic effects on oxidized LDL and remnant lipoproteins in plasma. Clinica Chimica Acta, 367: 36-47.
- Maurizio A, Ulisse G, Pierfrancesco A, Massimiliano F, Anna F, Cristina 2006. Plasma levels of oxidized-low-density lipoproteins are higher in patients with unstable angina and correlated with angiographic coronary complex plaques. Atherosclerosis, 185: 114-120.
- Mahdavi R, Pak Nahad M, Askari S. et al. Effects of olive / cholesterol diet on lipoprotein serum, lipid peroxidation and atherosclerosis creation in rabbits. Research in medicine. 2001-2002. 8(1): 15-19.
- Holman RL, Mcgill Hc Jr, Stong JP, Geer JC. 1998. Techniques for studies atherosclerotic lesion. Lab Inves, 7: 42-47.
- Morel DW, Dicorleto PE, Chisolm 1984. Endothelial and smooth muscle cells alter low density lipoprotein in vitro by free radical oxidation. Arteriosclerosis, 4(4): 357-64.
- Rober W, Erid S, Ton J 1998. Nitric oxide and hypercholestrolemia a matter of oxidation and reduction. Atherosclerosis, 137: 651-60.
- El-Arab AE, Foheid S, El-said M. Effect of yeast and botanical ?-glucan on serum lipid profile and cecum probiotic bacteria using rats fed cholesterol diet. Journal of Food and Nutrition Sciences 2009; 59 (2): 169- 174.
- Raafian M. The effect of ascorbic acid on hyperlipidemia. Journal of Shahrekord’s University of Medical Science. 3(2(1-5):1-5.
- Byers T, Perry 1992. Dietary carotenes, vitamin C and vitamin E as protective antioxidants in human cancers. Annu. Rev. Nutr, 12: 139-159.
- Forghani BM, Goharian V, Kasaiyan N, Amini M. Investigating on effects of vitamin C supplements on serum lipoproteins in patients with non-insulin dependent diabetes. Scientific Journal of Medical Association of Islamic Republic of Iran. 19(2): 95-100.
- Gürbüz I , Akyüz C, Yeşilada E , Şener B. Antiulcerogenic effect of Momordica charantia L. fruits on various ulcer models in rats. J Ethnopharmacology 2000; 71: 77-82.
- Khan IA, Abourashed EA. Leung’s encyclopedia of common natural ingredients: used in food, drugs and cosmetics. New York: Wiley; 2002
- Najafian M, Rahmanian E, Ahmadlou A, and et al. 2012. Investigation of Serium Lipids Level in Hemodialysis Patients in a Referral Center. Archive des sciences, 65 (7): 681- 688.
- Shojaei S, Kargar Jahromi H, Kohpeyma F, Bathaee SH, Farzam M. 2015. The comparison of pathophysiological effects of the extract of Trigonella and Galega plant with metformin on some biochemical parameters in streptozotocin- induced diabetic rat. Comp clin pathol, 24: 303- 309.
- Rojhan S. Basic Human Histology. Chehreh Publication. 1999-2000, 147-150.
- Lichtenstein AH, Ausman LM, Carrasco W, et al. 1994. Hypercholesterolemic effect of dietary cholesterol in diet enriched in polyunsaturated and satirated fat. Arterioscler thromb, 14: 168- 75.
- Abd albari A. Tazkaratoldavod. 1st ed. Dar altalae. Qahere. 2001, pp: 138.
- Alantaki D. Boghyat Almohtaj fee Almojarrab men Alaj. 1st ed. Darelfikr. Lebanon. 2001, pp: 224, 226.
- Norani M. The Great Encyclopedia of Islamic Medicine. 1st ed. Armaghane Yosef. Qom. 1384, vol. 1, pp: 231








