Farokhnia Farzaneh*, Hosseini Seyed Ebrahim, Vahdati Akbar
Department of Biology, Science and Research Branch, Islamic Azad University, Fars, Iran. Corresponding Author: Email: Farokhnia1372@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/611
Abstract
azathioprine is a chemical drug that is used for treatment of diseases with immunological origin. Studies have proven that the drug has potential side effects on the renal tissues. So finding a suitable antioxidant to reduce the pathological effects of azathioprine drug on kidney is the objective of the present study. Methods: the study was conducted on 42 mature female rats. After weighing, the rats were divided into 6 groups of 7 each, which includes control (without any treatment), blank (received 5 mg ofnormal saline), experimental group1 (azathioprine (50mg/kg) were administered intraperitoneally), experimental group2 (received azathioprine (50mg/kg) intraperitoneally and orally received wormwood extract (125mg/kg)), experimental group3 (received azathioprine (50mg/kg) intraperitoneally and orally received wormwood extract (250mg/kg)), experimental group4 (received azathioprine (50mg/kg) intraperitoneally and orally received wormwood extract (500mg/kg)). After 28 days, the rats of all groups became unconscious and their renal tissues were isolated for histological studies. Results: the results showed that reduction of glomerular and collecting ducts diameter, as well as malpighian body diameter were observed at the level of 5% in the experimental group1 in compared to control. The collecting ducts diameter in the experimental group3,which had received the maximum dose of the extract,increased compared to the experimental group 1. Conclusion: the obtained results of the study show that azathioprine drug causes impairment in renal tissues and wormwood extract due to its antioxidant properties, reduces the pathological effects of azathioprine.
Keywords
Wormwood; Histopathologic; Kidney; Azathioprine; Rat
Download this article as:| Copy the following to cite this article: Farzaneh F, Ebrahim H. S, Akbar V. Investigating on Effect of Wormwood Extract on Reduction of Renal Toxicity in Treated Rats by Azathioprine. Biomed Pharmacol J 2015;8(1) |
| Copy the following to cite this URL: Farzaneh F, Ebrahim H. S, Akbar V. Investigating on Effect of Wormwood Extract on Reduction of Renal Toxicity in Treated Rats by Azathioprine. Biomed Pharmacol J 2015;8(1). Available from: http://biomedpharmajournal.org/?p=839 |
Introduction
Since renal diseases are one of the major problems in today’s societyso careful attention to their structure and function can have an important role in individual health. Drugs and chemicals consumption increases day by day but some of their side effects such as autoimmune and resistance are created by continuous, indiscriminate and arbitrary consumption of drugs. Long-term use of drugs and in some cases cut ofdrugs’ consumption, causes additional side effects that may be more dangerous than the disease itself. Although consumption of synthetic drugs has been developed due to their successful effects, but production problems, high cost, and side effects have forced researchers to study and investigate side effects of these drugs on different parts of body (1). One of these drugs is azathioprine that has been used since 1960 and is known by some trade name such as Azamun, Percaprine, and Immunoprin. Azathioprine is used for treatment of some diseases such as leukemia (2, 3), rheumatoid arthritis (4), acute lymphoblastic (5), inflammatory intestine (6), and stratiev colitis (4, 7-9). On the other hand, azathioprine in addition to suppressing of lymphocytes activity in patients (10)also causes toxicity in bone marrow, liver, and gastrointestinal tract (7). It is found that the drug can lead to organs’ toxicity by creation of an oxidative lesion. The toxicityis occurred so that free radicals and oxidative stress in body organs and tissues are produced by azathioprine. So creation of oxidative lesion is one of the important factors for creation of organs’toxicity. So, most of sciences are developed to largely reduce chemical effects of drugs. One of the sciences is knowledge of using medicinal plants. Today, in addition to chemical drugs, herbal medicines are also used for treatment of diseases (11). One of these medicinal plants is wormwood (12) by scientific name of Artemisia absinthium.Wormwood is from Chicory family (13).Its essence contains several compounds such as cis chrysanthenyl acetate, cis chrysanthenone, cis epoxy ocimene, sabinile acetate, burnil acetate and α- and β-thujone (14-16).
Studies have shown that the amount of α- and β-thujone in wormwood is depends on the area where the plant growth. So that in some countries thujone amount is very high and in others, such as France (17), Italy (18), Spain (19), and Egypt (20), there are some reports based on lack of existence of thujone in grown plant in those areas. It is reported that flowers of wormwood plant in Iran have low β-thujone and do not have any α-thujone, so toxicity of this plant is low and it can be used readily for consumption in traditional medicine as well as food industries (21).
Wormwood has some properties such as, anti-worm effects, creation of menses and bile, respiratory, stomach nourishing, anti-fever, disinfectant and anti-poisoning to lead. Also the plant is digestive and stomach tonic, and is anti-scrofula, anti-jaundice, anti-dropsy and anti-crippling arthritis (13).
Due to adverse effects of azathioprine on renal tissues and wormwood extract antioxidant effects and due to the fact that no research has performeduntil now on the effect of wormwood plant on renal tissues of rats treated by azathioprine; so the main purpose of the study is investigation on the effect of wormwood extract on renal tissues of treated rats by azathioprine.
Methods
In the study, 42 healthy female Wistar rats which were prepared from Animals House in Yasouj University of Medical Science were used and they were kept in the House for two weeks to adapt to environment. The animals had 2 month and weighted about 160-180g. They all had free access to food and water.
The animals were randomly divided into six groups of seven each, as follows:
Control: rats were kept in a normal mode, without receiving any drugs.
Blank: they were intraperitoneally received 5ml of normal saline solution (9%) for 28 days.
Experimental 1: they received 50mg/kg of azathioprine, without receiving any extract for 28 days.
Experimental 2: they were intraperitoneally received 50mg/kg of azathioprine, and orally received 125 mg/kg.BW of wormwood extract for 28 days.
Experimental 3: they were intraperitoneally received 50 mg/kg of azathioprine, and orally received 250 mg/kg.BW of wormwood extract for 28 days.
Experimental group 4: they were intraperitoneally received 50 mg/kg of azathioprine, and orally received 500 mg/kg.BW of wormwood extract for 28 days.
Preparation of hydro-alcoholic wormwood extract
To prepare wormwood extract, the plant roots were primarily powdered with mill and Soxhlet method was selected in continuous. In this method, for every 10 g of wormwood powder related amount of 200 ml of solution containing water and alcohol was added to it. The obtained solution was placed in Soxhelt device, and then Rotavapor device removed solution from the extract (22).
To determine dosage of drug and extract, according to articles and previous experiences in laboratory and in the present study, half of the rats died after administration of the extract at a dose of 1000 mg/kg; also, half of the rats died after administration of azathioprine at a dose of 100 mg/kg.BW. So, obtainedwormwood LD50 is 1000 mg/kg.BW and lethal dose of azathioprine is 100 mg/kg.BW.
After completion of 28-day period, all rats weighted and became unconscious by ether. Their renal tissues were extracted and sent to laboratory for other stages and preparation of lamina. One-way Analysis of Variance (ANOVA) for comparison between treatments followed by t-test and Ducan test was used for multiple comparisons between groups. P<0.05 was considered as a significant level. Data analysis and statistical testing was performed using SPSS, version 18.
Results
The results obtained from measuring diameter of malpighian body show a significant reduction at level of 5%for the experimental group 1 in compared to control. Other experimental groups do no show significant difference compared to control (Chart 1).
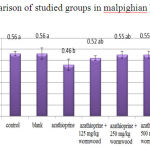 |
Figure 1: Comparison of studied groups in malpighian bodydiameter |
The obtained results of renal glomerular diameter measurement show a significant reduction at level of 5% for the experimental group 1 in compared to control (Chart 2).
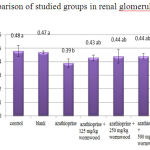 |
Figure 2: Comparison of studied groups in renal glomerular diameter
|
The obtained results of renal capsule-diameter measurementdo not show a significant difference for all groups compared to control (Chart 3).
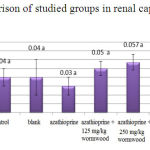 |
Figure 3: Comparison of studied groups in renal capsule-diameter |
The obtained results of proximal tubule-diameter measurement among different groups do not show a significant difference for all groups compared to control (Chart 4)
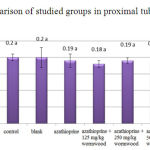 |
Figure 4: Comparison of studied groups in proximal tubule-diameter |
The obtained results of distal tubule-diameter measurement do not show a significant difference at level of 5% for all groups compared to control (Chart 5)
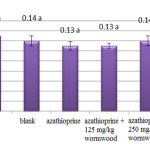 |
Figure 5: Comparison of studied groups in distal tubule-diameter |
The obtained results of loop of Henle-diameter measurement do not show a significant difference for all groups compared to control (Chart 6).
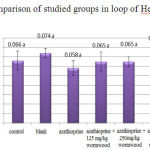 |
Figure 6: Comparison of studied groups in loop of Henle-diameter |
The obtained results of urine collecting ducts-diameter measurement show a significant reduction for experimental groups 1 and 2 compared to control. Also, these results of urine collecting ducts-diameter measurement do not show a significant difference at level 5% for the experimental group 4 compared to the experimental group 1(Chart 7).
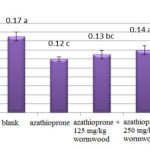 |
Figure 7: Comparison of studied groups in urine collecting ducts |
The available mean in each row that at least has a letter in common do not have a significant difference at level of 5%,based on Duncan test.
Histology results indicate that renal tissue in control and blank was normal and no tissue damage was observed (Figure 1 and 2).
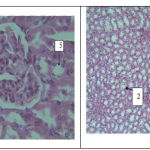 |
Figure: 8 |
Figure 1(left)- a photo-micrograph of kidney tissue in control group. 1: malpighian body, 2: glomerulus, 3: capsule, 4: proximal tubule, 5: distal tubule.Magnification is40 and it was stained by Hematoxylin- eosin.
Figure 2 (right)- a photo-micrograph of kidney tissue in blank group. 1: proximal tubule, 2: distal tubule. Magnification is 4 and it was stained by Hematoxylin- eosin.
According to the results, reduction in proximal tubule diameter as well as damage in endothelial capillaries of glomerulus is observed in the experimental group 1, which received azathioprine (Figure 3)
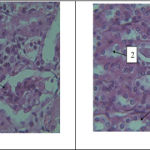 |
Figure 9 |
Figure 3 (left)- a photo-micrograph of kidney tissue changes in the experimental group1. 1: glomerular damage. Magnification is 40 and it was stained by Hematoxylin- eosin.
Figure 4 (right)- a photo-micrograph of comparison of diameter in. 1: distal tubule, 2: proximal tubule, 3: urine collecting ducts (reduction) of kidney tissues in experimental group2. Magnification is 40 and it was stained by Hematoxylin- eosin.
The results of renal tissue in the experimental group 2 which received azathioprine with low-dose of extract show that urine collecting ducts diameter is reduced compared to control. Also tissue damage in urine collecting duct (Figure 4) as well as glomerulus and distal and proximal tubules damage (Figure 5) of the experimental group 2 is observed compared to control
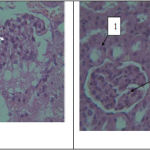 |
Figure 10 |
Figure 5 (left)- a photo-micrograph of kidney tissue changes in the experimental group2. 1: glomerular damage. Magnification is 40 and it was stained by Hematoxylin- eosin.
Figure 6 (right)- a photo-micrograph of comparison of diameter in. 1: distal tubule, 2: glomerular changes of kidney tissues in experimental group3. Magnification is 40 and it was stained by Hematoxylin- eosin.
Histology results in experimental group 3 show renal tissue damage is less than renal duct damage in control group,although the results show that histological changes of this group is not significant compared to control (Figure 6). Reduction of tissue damage and a small amount of hyperemia compared to groups which received azathioprine is also observable in Figure 7.
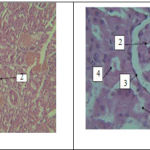 |
Figure 11 |
Figure 7 (left)- a photo-micrograph of kidney tissue changesof. 1: hyperemia, 2: glomerular damage, 3: ducts damages in experimental group3. Magnification is 10 and it was stained by Hematoxylin- eosin.
Figure 8 (right)- a photo-micrograph of kidney tissue changes of 1: malpighian body, 2:glomerulus, 3: capsule, 4:distal tubule, 5: proximal tubule,in experimental group4. Magnification is 40 and it was stained by Hematoxylin- eosin.
Histology results indicate that increase diameter of malpighian body as well as collecting ducts is observable in the experimental group 4 that received high-dose of extract. Tissue damage is less than the experimental group 1, 2 and 3, which represents positive effects of the extract on renal tissue changes in groups receiving azathioprine (Figure 8, Figure 9).
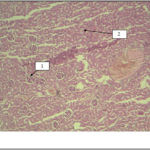 |
Figure 12 |
Figure 9- a photo-micrograph of kidney tissue changes in experimental group4, 1: glomerulus, 2: renal ducts. Magnification is 4 and it was stained by Hematoxylin- eosin.
Discussion
The results show that malpighian body and glomerulardiameter in the experimental group 1 (received azathioprine alone) has significant reduction compared to control and blank. Urine collecting duct diameter in the experimental groups 1 and 2 has also significant reduction at 5% level compared to control.
It is stated in research done in the past that use of azathioprine lead change and damage to some part of bone marrow cells, and therefore number of blood cells is reduced (23). In investigation on the effects of azathioprine on renal tissue in kidney disease, it is stated that the drug reduces somewhat glomerulardiameter (24). Another study suggested that azathioprine causes adventitious and structural changes in cell, fragmentation of chromosome, reduce mitotic division and increase the number of cells that have multiple chromosomes (25).
It has also found that the drug can cause impairment by creation of an oxidative lesion (26, 27). In past studies observed that azathioprine causes production of free radicals in body’s organ and tissues. As it is proved,free radicalis one of the main factors of causing body’s toxicity (28). In another past research stated that azathioprine drug can prevent synthesis of purine bases and prevent re-replication in cells. This action results in lack of synthesis of DNA and RNA. Toxicity resulting from the use of azathioprine is also proven in some organs such as marrow bone, pancreas, and liver (7, 26). So, in the present study, reduce of diameter of urine collecting ducts, malpighian body, glomerulus in groups that received azathioprine is quite reasonable compared to control and blank. This indicates the harmful effects of the drug on different parts of renal tissue.
In performed studies on lesion leads to impairment in renal function stated that any impairment in normal urine flow and its resulted consequences is called obstructive uropathy.In Urology perspective, construction and stop of urine in the path of collecting ducts has great importance in damage to kidney function. Any change can finally lead to hydronephrosis, atrophy and even complete destruction of kidney function. In addition, these changes can cause infection and so damages induced by these changes will be doubled. The effects of obstruction on kidney function are important for prediction and treatment of obstruction. The exact mechanisms of kidney changes are not exactly known and are considered by many researchers (29, 30). Researchers stated that increase of pressure in blocked part causes increase of back pressure and direct damage to renal parenchyma also can cause several impairments such as disorder in oxidative balance, energy metabolism, hemodynamic and renal excretory function (31). Hyperuricemia may be associated with pre-glomerular vascular damage, glomerular hypertension, reduction of renal perfusion and can cause localized fibrosis (32-34).
The studies found that azathioprine causes glomerular lesions and reduces its diameter (35). So changes related to renal different ducts particularly changes in glomerulus are very important and any changes in them cause impairment in kidney function and destruction of renal tissues. On the other hand, it is stated that azathioprine inhibits DNA synthesis in different organs such as liver, pancreas, duodenum, lymph nodes,and kidneys and also it is a causing factor of oxidative stress (7, 26). Watanabe et al.proved that oral administration of azathioprine leads to cell and rough endoplasmic reticulum necrosis and mitochondria proliferation (36). This also in itself causes damage to renal tissues. Also in the present study reduction of glomerular diameter as well as collecting ducts and malpighian body may be a ground for subsequent damages such as hyperuricemia and damage to renal parenchyma. The results show that in the experimental group 4 collecting ducts diameter is increased compared to the experimental group 1 which has received azathioprine alone. This reflects positive effect of the extract. Distal and proximal tubule, loop of Henle,malpighian body and glomerular diameters in the experimental groups which have received the extract associated with azathioprine are increased compare to the experimental group 1 that received azathioprine alone. Although these changes are not statistically significant at level of 5%, they cannot be ignored and represent the positive effect of the extract.
Traditional medicine and herbal drugs are now the most popular method of treatment. Wormwood is a drug that received less attention. It has anti-radical activity which depends on the amount of concentration also its activity depends to its flavnoids and phenolic compounds (37).
As mentioned above one of the causes of reduction in glomerular, malpighian body and urine collecting ducts diameters in the experimental group 1 which received azathioprine alone,is increase in active species of oxygen. So it is likely that wormwood by having flavonoid compounds and antioxidant properties reduce harmful effects of azathioprine in renal tissues.
Studies suggest that wormwood has protective effect in liver and kidney against lead’s effects. And cholesterol, triglyceride,fatty acids, and phospholipids levels are also reduced thereby reduce lipid peroxidation (38). Other studies have also stated that wormwood has anti-inflammatory activity (39). Also past studies showed that thujone essence presence in wormwood is used in herbal medicine to treat various diseases (13, 40). In the current study, it is likely that wormwood extract by having anti-oxidant and flavonoid compounds improve renal tissue in the groups which treated by azathioprine.
Conclusion
According to the performed research can be conclude that azathioprine may cause destructive effect on kidney tissues especially on glomerulus, malpighian body and urine collecting ducts and wormwood extract reduces these effects to some extent. So wormwood extract can be used as an antioxidant to reduce risks of azathioprine.
References
- Velog J. Estado J.Medicinal plants. Translated by: Zaman S.,Publication of Ghoghnoos., third edition. 1997-1998, pp 96-99, 108-150.
- Aarbakke J, Janka-Schaub G, Elion GB. Thiopurine biology and pharmacology. Trends Pharmacol Sci.1997; 18(1): 3-7.
- Czaja Aj. Drug therapy in the management of type 1 autoimmune hepatitis. Drugs. 1999; 57(1): 49-68.
- Kerstens PJ, Boerbooms AM, Jeurissen ME, de Graaf R, Mulder J, van de Putte LB. Radiological and clinical results of longterm treatment of rheumatoid arthritis with methotrexate and azathioprine. J Rheumatol.2000; 27(5): 1148-55.
- Ludwig D, Stange EF. Efficacy of azathioprine in the treatment of chronic active Crohn’s disease:prospective one-year follow-up study. German Imurek Study Group. Z Gastroenterol 1999; 37(11): 1085-91.
- Pearson DC, May GR, Fick G, Sutherland LR. Azathioprine for maintaining remission of Crohn’s disease.Cochrane Database Syst Rev 2000; (2): CD000067.
- Sweetman SC, Blake PS, Parsons AV (eds). Martindale’s In: the Complete Drug Reference. 33rd Ed.London: Pharmaceutical Press; 2002. p. 470–572.
- Mattheus CB Wielenga, Jooske F van Lidth de Jeude, Sanne L Rosekrans, and et al. 2014. Azathioprine does not reduce adenoma formation in a mouse model of sporadic intestinal tumorigenesis. World J Gastroenterol. 28; 20(44): 16683-16689.
- Kader HA, Mascarenhas MR, Piccoli DA, Stouffer NO, Baldassano RN. Experiences with 6-mercaptopurine and azathioprine therapy in pediatric patients with severe ulcerative colitis. J Pediatr Gastroenterol Nutr 1999; 28(1): 54-8.
- Di landro D, Sarzo G, Marchini F. New immunosuppressive treatment in kidney transplantation. Clin Nephrol 2000; 53(4): suppl 23-32.
- Sohyli khah S, Salman Roghani H, Mohammadi S M. Report of a cholestatic hepatitis due to consumption of medicinal plant called Teucrium. Journal of Medical Science and Health Services of Shahid Sodughi, Yazd, 2007-2008. 15 (3): 97-99.
- Ramezani M, Fazli-Bazzaz BS, Saghafi-Khadem F, Dabaghian A. Antimicrobial activity of four Artemisia species of Iran. Fitoterapia. 2004; 75(2): 201-3.
- Gholami M, Azizi A. The effect of nitrogen fertilizer on total amount of essence and values of α- thujone and chazoline on Artemisia absinthium wormwood. Agricultural research of water, soil and plant in agriculture, 2006-2007, 6(3): 83-93.
- Fleming Th. PDR for Herbal Medicines. 1st ed. Medical Economics Company. Montvale. 1998, pp: 664 – 5.
- Scott TL, Buhner SH. Invasive Plant Medicine: The Ecological Benefits and Healing Abilities of Invasive. Healing arts press, 2010.
- Hoffmann D. Medical herbalism: the science and practice of herbal medicine. Healing arts press, 2003, 246-253.
- Padosch SA, Lachenmeier DW and Kroner LU. Absinthism: a fictious 19th century syndrome with present impact. Subst. Abuse Treat. Prev. Policy. 2006; 1 – 14.
- Juteau F, Jerkovic I, Masotti V, Milos M, Mastelic J, Bessiere JM and Viano J. Composition and antimicrobial activity of the essential oil of Artemisia absinthium from Croatia and France. Planta Med. 2003; 69: 158 – 61.
- Nin S, Arfaioli P and Bosetto M. Quantitative determination of some essential oil components of selected Artemisia absinthium plants. J. Essent. Oil Res. 1995; 7: 271 – 7.
- Arino A, Arberas I, Renobales G, Arriaga S and Dominguez JB. Essential oil of Artemisia absinthium L. from the Spanish Pyrenees. J. Essent. Oil Res. 1999; 11: 182 – 4.
- Aboutabl EA, El Azzouny AM and El Dahmy SI. Constituents of the essential oil of Artemisia absinthium grown in Egypt. J. Essent. Oil Bear Plants. 2003; 6: 41 – 5.
- Samsam SH. Collection of medicinal herbs. 1st Tehran: char bagh, 2007. p. 938.
- Meier-Kriesche H, Vaghela M, Thambuganipalle R, Friedman G, Jacobs M, Kaplan B. 1999. The Effect of Body Mass Index on Long-Term Renal Allograft Survival. Clinical Transplantation, 68 (9): 1294- 1297.
- Weldon D, Shelp JMB, Bloodworth Jr, Richard E, Rieselbach MD. 1991. Effect of Azathioprine on Renal Histology and Function in Lupus Nephritis. Arch Intern Med, 128 (4): 566- 573.
- Fardous SK, Abeer FEN. 2006. The protective effect of vitamin C on Azathioprine induced seminiferous tubular structural changes and cytogenetic toxicity in albino rats. Cancer Therapy, 4: 125- 134.
- Lee AU, Farrell GC. Mechanism of azathioprine-induced injury to hepatocytes: roles of glutathione depletion and mitochondrial injury. J Hepatol 2001; 35(6): 756-64.
- Sood A, Midha V, Sood N, Kaushal V. Role of azathioprine in severe ulcerative colitis: one-year, placebocontrolled, randomized trial. Indian J Gastroenterol 2000; 19(1): 14-6.
- Park SW, Lee SM. 2008. Antioxidant and prooxidant properties of ascorbic acid on hepatic dysfunction induced by cold ischemia/reperfusion. Eur J Pharmacol, 580(3): 401-6.
- Klahr S, Pukerson ML. 1994. The pathophysiology of obstructive nephropathy, The role of vasoactive compounds in the hemodynamicand structural abnormalities of the obstructed kidney. Am J Kidney Dis, 23:219-23.
- Klahr S. 1991. New insights into the consequences and mechanisms of renal impairment in obstructive nephropathy. Am J Kidney Dis, 18:689-99.
- Vaughan ED, Marion D, Poppas DP. Felsen D. 2004. Pathophysiology of unilateral ureteral obstruction: studies from Charlottesville to New York. J Urol, 172(6 Pt 2):2563-9.
- Sanchez-Lozada LG, Tapia E, Santamaria J, Avi- la-Casado C, Soto V, Nepomuceno T, et al. 2005. Mild hyperuricemia induces vasoconstriction and maintains glomerular hypertension in normal and remnant kidney Kidney Int, 67(1): 237- 47.
- Nagahama K, Inoue T, Iseki K, Touma T, Kinjo K, Ohya Y, et al. 2004. Hyperuricemia as a predictor of hypertension in a screened cohort in Okinawa, Japan. Hypertens Res, 27(11): 835-41.
- Kang DH, Nakagawa T, Feng L, Watanabe S, Han L, Mazzali M, et al. 2002. A role for uric acid in the progression of renal disease. J Am Soc Nephrol, 13(12): 2888-97.
- Hollander AAMJ, van Saase JLCM, van Es LA, van derWoude FJ, van Bockel HJ, et al. 1995. Beneficial effects of conversion from cyclosporin to azathioprine after kidney transplantation. The Lancet, 345 (8950): 610- 614.
- Watanabe A, Hobara N, Tobe K, Endo H, Nagashima 1997. Biochemical and morphological study on hepatotoxicity of azathioprine in rat. Acta Med Okayama, 33(1): 5-14.
- Cnadanovic JM, Djilas S, Gordana SC, Vesna TT. Free-radical scavenging activity of wormwood (Artemisia absinthium L) extracts. Journal of the Science of Food and Agriculture, 2005; 85 (2): 265-272.
- Kharoubi, O, Slimani, M, Aoues, A, Seddik L. Prophylactic effects of Wormwood on lipid peroxidation in an animal model of lead intoxication. Indian J Nephrol. 2008; 18(2): 51–57.
- Fayaz A, Rafeeq AK, Shahid R. Study of analgesic and anti inflammatory activity from plant extracts of lactuca scariola and Artemisia absinthium. Pharmacology, 1992; 5 (2): 111- 114.
- Wake G, Court J, Pickering A, Lewis R, Wilkins R, Perry E. CNS acetylcholine receptor activity in European medicina plants traditionally used to improve failing memory. Journal of Ethno Pharmacology 2000; 69(2): 105-114.








