Vahid Behnod1 , Hamid Yahaghi2 , Mohammad Hesam Sohani3 , Hoda Dezhkhi4*
1Baqiyatallah University of Medical Sciences , Tehran , Iran 2Young Researchers and Elite Club, North Tehran Branch, Islamic Azad University, Tehran, Iran 3Department of Microbiology, Science and Research Branch, Islamic Azad University, Tehran, Iran 4Department of Microbiology, Damghan Branch, Islamic Azad University, Damghan, Iran Corresponding Author Email : hodadezhkhi@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/596
Abstract
The present investigation was carried out to study the prevalence of Staphylococcus aureus and its antimicrobial resistance pattern isolated from various types of human clinical infections. One hundred and fifty clinical specimens were collected from the educational hospitals of Iran. Samples were cultured and those that were S. aureus-positive were confirmed using the PCR. Antimicrobial resistance pattern was analyzed using the disk diffusion method. Of 150 samples studied, 50 samples (33.33%) were positive for S. aureus. The results of the culture method were confirmed using the PCR amplification of 16S rRNA gene of the S. aureus. Burn infections had the highest prevalence of S. aureus (40%). S. aureus strains of our study harbored the highest levels of resistance against penicillin (94%), tetracycline (92%), oxacillin (90%) and azithromycin (80%). Keep the skin and especially wound and burn infections clean away from the polluted environment of hospitals and regular prescription of imipenem, methicillin and vancomycin may be good instructions to reduce the risk of S. aureus infections in the cases of human clinical infections.
Keywords
Staphylococcus aureus; Detection; Antibiotic resistance properties; clinical infections; Iran
Download this article as:| Copy the following to cite this article: Behnod V, Yahaghi H, Sohani M. H, Dezhkhi H. HDetection and Antimicrobial Resistance Properties of Staphylococcus Aureus Strains Isolated from the Human Clinical Infections. Biomed Pharmacol J 2015;8(1) |
| Copy the following to cite this URL: Behnod V, Yahaghi H, Sohani M. H, Dezhkhi H. HDetection and Antimicrobial Resistance Properties of Staphylococcus Aureus Strains Isolated from the Human Clinical Infections. Biomed Pharmacol J 2015;8(1). Available from: http://biomedpharmajournal.org/?p=1595 |
Introduction
Staphylococcus aureus (S. aureus) has long been deliberated as a main pathogen of hospital infections all-around the world. It is a bacterium that frequently colonizes the human skin. The bacterium can exist in this form without harming its host or causing symptoms. However, if there is a break in someone’s skin from a wound, burn or surgery, or if there is a suppression of a person’s immune system, then colonizing S. aureus can cause an infection. It is documented that the S. aureus is one of the most routine causes of skin and soft-tissue infections and especially superficial wounds, post-surgical wounds and burn infections (1-5). S. aureus may also infect others as it can be passed from both infected and colonized people to other people through skin contact or through sharing contaminated objects, such as towels or razors. Therefore, consideration of the S. aureus as a threating pathogens for human health especially in the cases of skin infections has critical health importance (6).
The ability of S. aureus to resistance against wide range of antibiotics causing severe problems in treatment of hospital infections. S. aureus has developed resistance to multiple classes of antibiotics, especially beta-lactams. It has been documented that majority of S. aureus strains were resistant to sulfamethoxazole-trimethoprim, erythromycin, oxacillin, ampicillin, penicillin, tetracycline, chloramphenicol, cotrimoxazole, gentamicin, cefexim and clindamycin (7, 8). In recent years prevalence of resistance against methicillin and vancomycine has been increased throughout the world (9-13).
Materials and Methods
Ethical consideration
The present study was accepted by the ethical committees of the educational Hospitals. Written informed consent was obtained from all of the study patients or their parents.
Samples collection
Overall 150 clinical samples from various types of infections including superficial wound (n=50), post-surgical wounds (n=50) and burn infections (n=50) were collected from hospitalized patients of major educational hospitals of Tehran, Iran. All samples were immediately transferred to the laboratory at 4°C in a cooler with ice packs.
Staphylococcus aureus identification
All samples were directly cultured into 7% sheep blood agar (Merck, Darmstadt, Germany) and incubated aerobically at 37°C for 48 h. After incubation, suspicious colonies were examined by the use of morphologies compatible with Staphylococcus spp. (microscopical morphology, catalase and coagulase production). Studied colonies were cultured on Tryptic Soy Broth (TSB) (Merck, Darmstadt, Germany) and Tryptic Soy Agar (TSA) (Merck, Darmstadt, Germany). After growth, staphylococci were identified on the basis of colony characteristics, Gram staining, pigment production, hemolytic and the following biochemical reactions: catalyses activity, coagulated test (rabbit plasma), Oxidase test, glucose O/F test, resistance to bacitracin (0.04 U), mannitol fermentation on Mannitol Salt Agar (MSA) (Merck, Darmstadt, Germany), urease activity, nitrate reduction, novobiocin resistance, phosphatase, deoxyribonuclease (DNase) test and carbohydrate (xylose, sucrose, trehalose and maltose, fructose, lactose, mannose) fermentation test (14).
Antimicrobial susceptibility test
Pattern of antimicrobial resistance was studied using the simple disk diffusion technique. The Mueller–Hinton agar (Merck, Germany) medium was used for this purpose. Antibiotic resistance of S. aureus strains against 15 commonly used antibiotics in the cases of UTIs was determined using the instruction of Clinical and Laboratory Standards Institute guidelines (15). Susceptibility of S. aureus isolates were tested against ampicillin (10 u/disk), gentamycin (10 µg/disk), amikacin (30 u/disk), imipenem (30 u/disk), methicillin (30 µg/disk), tetracycline (30 µg/disk), vancomycine (5 µg/disk), norfloxacin (30 µg/disk), cotrimoxazole (30 µg/disk), clindamycin (2 µg/disk), trimethoprim–sulfamethoxazole (25 μg/disk), penicillin (10 u/disk), oxacillin (1µg/disk), erythromycin (15µg/disk), azithromycin (15 µg/disk) and cefexime (5 μg/disk) antibiotic agents (Oxoid, UK). The plates containing the discs were allowed to stand for at least 30 min before incubated at 35°C for 24 h. The diameter of the zone of inhibition produced by each antibiotic disc was measured and interpreted using the CLSI zone diameter interpretative standards (CLSI 2012) (15). S. aureus ATCC 25923 and Escherichia coli ATCC 25922 were used as quality control organism in antimicrobial susceptibility determination.
DNA extraction and PCR confirmation
Total genomic DNA was extracted from the bacterial colonies. A single colony was inoculated on 5ml of brain heart infusion broth and incubated over night at 37ºC. Then 1.5 ml of a saturated culture was harvested with centrifugation for 5 min. at 14,000 rpm. The cell pellet was resuspended and lysed in 200µl of lysis buffer (40 mM Tris-acetate pH 7.8, 20 mM sodium-acetate, 1 mM EDTA, 1% SDS) by vigorous pipetting. To remove most proteins and cell debris, 66 µl of 5M NaCl solution was added and mixed well, and then the viscous mixture was centrifuged at 12,000 rpm for 10min. at 4ºC. After transferring the clear supernatant into a new eppendorf tube, an equal volume of chloroform was added, and the tube was gently inverted at least 50 times when a milky solution was completely formed. Following centrifugation at 14,000 rpm for 5min., the supernatant is then removed to another eppendorf tube and double volume of 100% ethanol was added. The tubes were inverted 5 to 6 times gently, then centrifuged at 10,000rpm for 5minutes. The supernatant was discarded and 1ml of ethanol (70%) was added to the pellet, and tubes centrifuged at 10,000 rpm for 5 minutes. Finally the supernatant discarded and the pellet was dried for 10 min at room temperature, the pellet was resuspended by 100µl H2O. The stock was kept at -20ºC until use. The DNA concentration has been determined by measuring absorbance of the sample at 260 nm using spectrophotometer (16). Presence of S. aureus in each DNA samples was confirmed using the Daniel et al. (1994) (17) method. The PCR reaction mix consist of 1 X PCR buffer (10 mM Tris-HCl, pH 8.3, 50 mM KCl and 0.001% (w/v) gelatin) with 4 mM MgCl2, 250 mM of each nucleotide (deoxynucleoside triphosphate), 0.5 mM of each primer (F: 5’ GGAATTCAAAGGAATTGACGGGGGC -3’ and R: 5’- CGGGATCCCAGGCCCGGGAACGTATTCAC -3’) (479 bp size of product for 16S rRNA gene of the S. aureus), 4 ng of the molecular beacon and 4 U of Jumpstart Taq DNA polymerase (Fermentas, Germany).
Statistical analysis
Statistical analysis was performed using SPSS/16.0 software for significant relationships. The incidences of serogroups, virulence factors and antibiotics resistance properties of S. aureus isolated from various types of infectious samples were statistically analyzed. Statistical significance was regarded at a P value < 0.05.
Results
Total distribution of S. aureus in various types of human infections samples of Iranian educational hospitals is shown in table 1. Of 150 samples studied, 50 samples (33.33%) were positive for S. aureus. The results of the culture method were confirmed using the PCR amplification of 16S rRNA gene of the S. aureus (Figure 1). Burn infections had the highest prevalence of S. aureus (40%). Significant statistical analysis was found for the prevalence of S. aureus between superficial and burn infections (P < 0.05). Pattern of antibiotic resistance among the S. aureus strains of various types of clinical infections is shown in table 2. S. aureus strains of our study harbored the highest levels of resistance against penicillin (94%), tetracycline (92%), oxacillin (90%) and azithromycin (80%). Bacterial strains of our investigation harbored the lowest levels of resistance against imipenem (4%), methicillin (8%) and vamcomycine (10%). Significant statistical analysis was found for the prevalence of resistance between penicillin and vancomycine (P < 0.05), tetracycline and imipenem (P < 0.05), tetracycline and vancomycine (P < 0.05) and oxacillin and methicillin (P < 0.05).
Table 1: Distribution of Staphylococcus aureus in various types of human infections.
| Type of samples | No. samples collected | Positive strains (%) | PCR confirmation (%) |
| Superficial wound | 50 | 13 (26) | 13 (26) |
|
Post-surgical wounds |
50 | 17 (34) | 17 (34) |
| Burn infections | 50 | 20 (40) | 20 (40) |
| Total | 150 | 50 (33.33) |
50 (33.33) |
Discussion
The present study showed that the burn, post-surgical wound and superficial wound infections were infected with resistant strains of S. aureus. Our results showed that the prevalence of S. aureus in the surficial, post-surgical and burn infections of hospitalized patients of Iranian hospitals were 26%, 34% and 40%, respectively. The burn wound is considered as one of the major health problems in the world (18). In the present study, S. aureus was the most common isolate which is similar to other findings (19, 20). One possible explanation for the high prevalence of S. aureus in the clinical samples of patients of our study is the fact that the hospital environment is so contaminated and antimicrobial agents are prescribed in an irregular and impermissible manner. In a study which was conducted on Addis Ababa, Ethiopia on the burn wound infections (21), bacterial infection was observed in 95 out of 114 patients (83.3%) of which, 66 (69.5%) had S. aureus infection. Alebachew et al. (2012) (21) reported that most of the S. aureus strains of wound infections were sensitive to vancomycin, clindamycin, kanamycin and erythromycin, but highly resistant to penicillin. They showed that all isolates were multi drug resistant, and one isolate was resistant to all the tested drugs. In an investigation which was conducted on Ahvaz (22), results showed that 27.8% of wound and blood specimens were infected by Staphylococci and among these 60% were identified as methicillin resistant. Ekrami et al. (2010) (22) showed that the highest resistance percentage belonged to ciprofloxacin (81.2%) and then amikacin (81%), carbenicillin (64.6%) and gentamicin (64.3%). Momtaz and Hafezi (2014) (23) reported that of 132 clinical samples, 66 were positive for S. aureus. Superficial and surgical wounds had the highest incidence of S. aureus (66.12%), while blood samples had the lowest incidence (15.38%). They showed that the S. aureus isolates harbored the highest levels of antibiotic resistance against azithromycin (62.12%), tetracycline (57.57%) and erythromycin (54.54%) which was similar to our results.
Table 2: Susceptibility of Staphylococcus aureus strains of various types of clinical infections against commonly used antibiotics.
| Antimicrobial agents | Types of infections (%) | |||
| Superficial wound (13) | Post-surgical wounds (17) | Burn infections (20) | Total (50) | |
| Ampicillin | 2 (15.38) | 4 (23.52) | 6 (20) | 12 (24) |
| Gentamycin | 2 (15.38) | 3 (17.64) | 5 (25) | 10 (20) |
| Imipenem | – | – | 2 (10) | 2 (4) |
| Tetracycline | 7 (53.84) | 9 (52.94) | 30 (15) | 46 (92) |
| Vancomycine | 1 (7.69) | 1 (5.88) | 3 (15) | 5 (10) |
| Methicillin | 1 (7.69) | 1 (5.88) | 2 (10) | 4 (8) |
| Norfloxacin | 3 (23.07) | 5 (29.41) | 7 (35) | 15 (30) |
| Cotrimoxazole | 3 (23.07) | 4 (23.52) | 7 (35) | 14 (28) |
| Clindamycin | 2 (15.38) | 2 (11.76) | 5 (25) | 9 (18) |
| Trimethoprim–sulfamethoxazole | 3 (23.07) | 5 (29.41) | 10 (50) | 18 (36) |
| Penicillin | 5 (38.46) | 13 (76.47) | 19 (95) | 47 (94) |
| Oxacillin | 3 (23.07) | 15 (88.23) | 17 (85) | 45 (90) |
| Erythromycin | 2 (15.38) | 10 (58.82) | 12 (60) | 23 (46) |
| Azithromycin | 2 (15.38) | 8 (47.05) | 9 (45) | 40 (80) |
| Cefexime | 1 (7.69) | 2 (11.76) | 5 (25) | 35 (70) |
Infection is the most important problem in the treatment of burn patients. The bacteriology of burn wounds is often poly-microbial in nature, and the presence of multidrug-resistant organisms is often associated with more severe clinical manifestations and poor response to antimicrobial therapy. Antibiotic susceptibility patterns served as a useful guideline for choosing an appropriate antibiotic. The results of our investigation showed that the S. aureus strains of our study harbored the highest levels of resistance against penicillin (94%), tetracycline (92%), oxacillin (90%) and azithromycin (80%). In the other hand, the S. aureus strains of our study were susceptible to imipenem, methicillin and vamcomycine antibiotics. Similar results have been reported previously (21-23). Methicillin is one of the best choices for S. aureus clinical infections. The rate of resistance against this antibiotic was 8% in our study. Important role of methicillin resistant S. aureus (MRSA) as a causative agent of human clinical infections has been reported previously (21-25). High prevalence of antibiotic resistance in our study is maybe due to the fact that the prescription of methicillin is very high in Iranian health center and hospitals. In addition, this finding showed that the Iranian hospital’s environments are so infected. Also, our results showed that antibiotics were used in a highly irregular manner in Iranian hospitals.
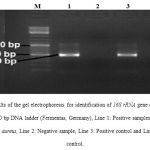 |
Figure 1: Results of the gel electrophoresis for identification of 16S rRNA gene of the S. aureus strains. M: 100 bp DNA ladder (Fermentas, Germany), Line 1: Positive samples for 16S rRNA gene of the S. aureus, Line 2: Negative sample, Line 3: Positive control and Line 4: Negative control. |
High prevalence of MRSA in various types of clinical infections were also reported by Alghaithy et al., (2000) (61% in Saudi-Arabia) (26), Młynarczyk et al., (2001) (40% in Warszawie) (27) and Rijal et al., (2008) (56.1% in Pokhara) (28). Virdis et al., (2010) (29) showed that the prevalence of resistance of S. aureus against kanamycin, oxytetracycline and ampicillin were 28%, 16% and 12%, respectively. Deng et al., (2013) (30) reported that the high prevalence of resistance of the S. aureus against most commonly used antibiotics including naficillin, oxacillin, vancomycin and cefathiamidine. Nishijima and Kurokawa (2002) (31) showed that the prevalence of S. aureus resistance against penicillin, cephalosporins and clindamycin were 20 to 30%. They showed that the prevalence of resistance against gentamycin, erythromycin and methicillin were 55.2%, 39.6% and 21%, respectively. Kumar et al., (2011) (32) revealed that the S. aureus isolates of clinical infections were highly resistant to different antibiotics, i.e. 33.6% were resistant to oxytetracycline, 36.4% to streptomycin, 29.9% to gentamycin and 26.2% each to chloramphenicol, pristinomycin and ciprofloxacin which was similar to our results.
Conclusions
The results of the present investigation showed that the S. aureus is one of the most important cause of infections in superficial, post-surgical and burn wounds. Therefore, its accurate diagnosis of in hospitals, patients and health care units is an important need. Also the dissemination of MRSA strains with high resistance to different antibiotics in Iranian hospitals is a warning for patient’s public health. Accurate and continuous surveillance of antibiotic resistance patterns among S. aureus strains should be considered in pediatrics. We recommended prescription of imipenem, methicillin and vancomycin in a highly regular pattern for treatment of cases of superficial, post-surgical and burn wound infections.
References
- Mittal R, Aggarwal S, Sharma S, Chhibber S, Harjai K. Urinary tract infections caused by Pseudomonas aeruginosa: a minireview. J Infect Public Health. 2009;2(3):101-11.
- Yahaghi E, Imani Fooladi AA, Amin M, Mirnejad R, Nezamzade R, Amani J. Detection of Class I Integrons in Staphyloacoccus aurous Isolated From Clinical Samples. Iran Red Crescent Med J. 2014 Nov 10;16(11):e16234.
- Kucheria R, Dasgupta P, Sacks SH, Khan MS, Sheerin NS. Urinary tract infections: new insights into a common problem. Postgrad Med J 2005;81:83—6.
- Shaikh N, Morone NE, Bost JE, Farrell MH. Prevalence of urinary tract infection in childhood: a meta-analysis. Pediatr Infect Dis J. 2008 Apr;27(4):302-8.
- Megged O1. Staphylococcus aureus urinary tract infections in children are associated with urinary tract abnormalities and vesico-ureteral reflux. Pediatr Nephrol. 2014 Feb;29(2):269-72.
- Goetghebeur M1, Landry PA, Han D, Vicente C. Methicillin-resistant Staphylococcus aureus: A public health issue with economic consequences. Can J Infect Dis Med Microbiol. 2007 Jan;18(1):27-34.
- Campoccia D, Montanaro L, Baldassarri L, An YH, Arciola CR. Antibiotic resistance in Staphylococcus aureus and Staphylococcus epidermidis clinical isolates from implant orthopedic infections. Int J Artif Organs. 2005 Nov;28(11):1186-91.
- Onanuga A1, Awhowho GO. Antimicrobial resistance of Staphylococcus aureus strains from patients with urinary tract infections in Yenagoa, Nigeria. J Pharm Bioallied Sci. 2012 Jul;4(3):226-30.
- Schlager TA1. Urinary tract infections in children younger than 5 years of age: epidemiology, diagnosis, treatment, outcomes and prevention. Paediatr Drugs. 2001;3(3):219-27.
- Tokajian S, Haddad D, Andraos R, Hashwa F, Araj G. Toxins and Antibiotic Resistance in Staphylococcus aureus Isolated from a Major Hospital in Lebanon. ISRN Microbiol 2011; 2011: 812049.
- Mehndiratta PL, Bhalla P, Ahmed A, Sharma YD. Molecular typing of methicillin-resistant Staphylococcus aureus strains by PCR-RFLP of SPA gene: a reference laboratory perspective. Indian J Med Microbiol 2009; 27(2): 116-122.
- Albrecht VS1, Zervos MJ, Kaye KS, Tosh PK, Arshad S, Hayakawa K, Kallen AJ, McDougal LK, Limbago BM, Guh AY. Prevalence of and risk factors for vancomycin-resistant Staphylococcus aureus precursor organisms in Southeastern Michigan. Infect Control Hosp Epidemiol. 2014 Dec;35(12):1531-4.
- Goud R, Gupta S, Neogi U, Agarwal D, Naidu K, Chalannavar R, Subhaschandra G. Community prevalence of methicillin and vancomycin resistant Staphylococcus aureus in and around Bangalore, southern India. Rev Soc Bras Med Trop. 2011 May-Jun;44(3):309-12.
- Zmantar T, Chaieb K, Ben Abdallah F, Ben Kahla-Nakbi A, Ben Hassen A, Mahdouani K, et al. Multiplex PCR detection of the antibiotic resistance genes in Staphylococcus aureus strains isolated from auricular infections. Folia Microbiol (Praha) 2008; 53(4): 357-362.
- Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing; twenty-second informational supplement. M100-S21. Wayne Pa: CLSI; 2012.
- Sambrok, J.A. (2001). Molecular Cloning: A Laboratory Manual. pp: 2100. 3rd ed. Cold Spring Harbor Laboratory Press, New York.
- Daniel JG, James RU, Cynthia AG, David HP. Multiplex PCR for identification of methicillin-resistant staphylococci in the clinical laboratory. J Clin Microbiol 1994;32(7):1768–72.
- Zorgani A, Zaidi M, Ranka R, Shahen A. The pattern and outcome of septicemia in a burns intensive care unit.J Burns Dis.2002;15:179–172.
- Lesseva M, Hadjiiski G. Staphylococcal infections in the Sofia Burn Centre, Bulgaria.J Burns. 1996;22:279–272.
- Komolafe O, James J, Kalongolera L, Makoka M. Bacteriology of burns at the Queen Elizabeth Central Hospital, Blantyre, Malawi.J Burns. 2003;29:235–238.
- Tigist Alebachew, Gizachew Yismaw, Ayelegn Derabe, Zufan Sisay. Staphylococcus Aureus Burn Wound Infection among Patients Attending Yekatit 12 Hospital Burn Unit, Addis Ababa, Ethiopia. Ethiop J Health Sci. 2012 Nov; 22(3): 209–213..
- Alireza Ekrami, Alireza Samarbafzadeh, Mohammad Alavi, Enayat Kalantar, Farhad Hamzeloi. Prevalence of methicillin resistant Staphylococcus species isolated from burn patients in a burn center, Ahvaz, Iran. Jundishapur Journal of Microbiology (2010); 3(2): 84-91.
- Momtaz H1, Hafezi L. Meticillin-resistant Staphylococcus aureus isolated from Iranian hospitals: virulence factors and antibiotic resistance properties. Bosn J Basic Med Sci. 2014 Oct 5;14(4):219-26.
- Mehndiratta PL, Bhalla P, Ahmed A, Sharma YD. Molecular typing of methicillin-resistant Staphylococcus aureus strains by PCR-RFLP of SPA gene: a reference laboratory perspective. Indian J Med Microbiol 2009; 27(2): 116-122.
- Havaei SA, Azimian A, Fazeli H, Naderi M, Ghazvini K, Samiee SM, et al. Genetic Characterization of Methicillin Resistant and Sensitive, Vancomycin Intermediate Staphylococcus aureus Strains Isolated from Different Iranian Hospitals. ISRN Microbiol 2012; 2012: 215275.
- Onanuga A, Awhowho GO. Antimicrobial resistance of Staphylococcus aureusstrains from patients with urinary tract infections in Yenagoa, Nigeria. J Pharm Bioallied Sci. 2012 Jul-Sep; 4(3): 226–230.
- Brown PD, Ngeno C. Antimicrobial resistance in clinical isolates of Staphylococcus aureus from hospital and community sources in southern Jamaica. International Journal of Infectious Diseases (2007) 11, 220—225.
- Voss A, Milatovic D, Wallrauch-Schwartz C, Rosdahl VT, Braveny I. Methicillin-resistant Staphylococcus aureus in Europe. Eur J Micr Infect Dis 1994; 13: 50-5.
- Tokajian S, Haddad D, Andraos R, Hashwa F, Araj G. Toxins and Antibiotic Resistance in Staphylococcus aureus Isolated from a Major Hospital in Lebanon. ISRN Microbiol 2011; 2011: 812049.
- Deng JJ, Zhu JN, Yang CL, Shu M, Xiao GG, Su M, et al. Clinical distribution and drug resistance of Staphylococcus aureus isolated from hospitalized children. Sichuan Da Xue Xue Bao Yi Xue Ban 2013; 44(1): 159-161.
- Boyce JM, Papa E, Dickenson R, Medeiros AA. Failure of routine susceptibility tests to detect imipenem resistance among strains of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 1991; 35(7): 1495-1497.
- Tiwari HK, Sen MR. Emergence of vancomycin resistant Staphylococcus aureus (VRSA) from a tertiary care hospital from northern part of India. BMC Infect Dis 2006; 6: 156.








