S. Kumar1*, A. Yadav2
1Department of Pharmacology, Kalinga Institute of Medical Sciences, Bhubaneswar, Odisha 2Department of Pharmacology, MES Medical College, Perinthalmanna, Kerala. Corresponding Author Email: subirrims@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/637
Abstract
To study the efficacy of ethanolic extract of Holarrhena antidysenterica (EEHA) seeds in streptozotocin (STZ)-induced diabetic rats and to investigate the qualitative phytochemical present in the extract. The study also aims to evaluate acute and short-term general toxicity of the extract in albino rats. EEHA seeds were subjected to preliminary qualitative phytochemical investigations by using standard procedures. The extract (300 mg/kg p.o.) was screened for antidiabetic activity in STZ-induced diabetic rats (30 mg/kg, i.p.). Acute oral toxicity study for the test extract of the plant was carried out using OECD/OCED guideline 425. Phytochemical analysis of EEHA seeds revealed the presence of flavonoids, saponins, carbohydrates, steroids, tannins, and phenolic compounds. In acute toxicity study, no toxic symptoms were observed for EEHA up to dose 3000 mg/kg. Oral administration of EEHA for 28th days exhibited highly significant (P < 0.01) hypoglycemic activity. The data were analyzed using analysis of variance for inter group comparison one way ANOVA was followed on SPSS package. The observations confirm that EEHA has antidiabetic activity. It also warrants further investigation to isolate and identify the hypoglycemic principles of this plant so as to elucidate their mode of action.
Keywords
Antidiabetes activity; Holarrhena antidysenterica; streptozotocin
Download this article as:| Copy the following to cite this article: Kumar S, Yadav A. Comparative Study of Hypoglycemic Effect of Holarrhena Antidysenterica Seeds and Glibenclamide in Experimentally Induced Diabetes Mellitus in Albino Rats. Biomed Pharmacol J 2015;8(1) |
| Copy the following to cite this URL: Kumar S, Yadav A. Comparative Study of Hypoglycemic Effect of Holarrhena Antidysenterica Seeds and Glibenclamide in Experimentally Induced Diabetes Mellitus in Albino Rats. Biomed Pharmacol J 2015;8(1). Available from: http://biomedpharmajournal.org/?p=1519 |
Introduction
Diabetes mellitus is a group of metabolic disorder characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both. The chronic hyperglycemia of diabetes is associated with the long-term damage, dysfunction, and failure of various organs, especially the eyes, kidneys, nerves, heart, and blood vessels. It was estimated that the total number of people with diabetes in 2010 to be around 50.8 million in India, rising to 87.0 million by 2030. [1] The disease has become a real public health problem in the developing countries, where its prevalence is increasing steadily and adequate treatment is often expensive or unavailable.
Alternative strategies to the current modern pharmacological therapy of diabetes mellitus are urgently needed,[2] because of the inability of existing modern therapies to control all the pathological aspects of the disorder, as well as the enormous cost and poor availability of the modern therapies for many rural populations in developing countries. Plants used in traditional medicine to treat diabetes mellitus represent a valuable alternative for the control of this disease [3].
Holarrhena antidysenterica (HA) (L) (Apocynaceae) is a medicinal plant, found throughout the Indian subcontinent. Stem bark of the plant, commercially known as “kurchi”, has been extensively investigated due to its traditional use in the treatment of amoebic dysentery, diarrhea, asthma, bronchopneumonia and malaria. Stem bark and seeds of the plant are reported to contain a number of steroidal alkaloids, such as conanines, 3-aminoconanines, 20-aminoconanines, 3-aminopregnans, 3, 20-diaminopregnanes and their derivatives. Being the principle alkaloid, conessine is mostly studied for its anti-diarrhoel properties [4]. A new steroidal alkaloid was isolated and characterized, designated as holadysenterine, together with three known steroidal alkaloids, conessine, isoconessimine and kurchessine from the stem bark of HA. [5] There are few studies of different parts of this plant related to its hypoglycemic effect. Fifty percent ethanolic extract of fruit has shown hypoglycemic activity in rats [6]. Aqueous and alcoholic extract of its bark has reversed the hyperglycemic condition produced by streptozotocin injection [7]. Scientific data related to the antidiabetic efficacy of ethanolic extract of its seed in diabetic rats is lacking. Thus the present investigation sets out to study the anti-hyperglycemic activity of H.A seed extract in streptozotocin induced diabetic rats. The effect produced by the drug was compared with that of glibenclamide, a standard drug.
Materials and Methods
Preparation of plant extract
The seeds of Holarrhena antidysenterica (HA) were obtained locally. Seeds were washed and then dried in incubator at 390C for 4 days. It was finally powdered in grinder, filtered through a muslin cloth. Ethanolic extract (with 90% ethanol) of it were made in the soxhlet apparatus. The extract was concentrated in evaporator under reduced pressure at 40˚C. The extract was stored at 0-4˚C.
Experimental Animals
Male albino rats of wistar strains weighing 150-200 g, and maintained in an air-conditioned room (25 ± 3°C) with a 12 hour light and dark cycle as per CPCSEA guidelines [8]. Feed and water were provided ad libitum. The experimental study was approved by the Institutional Ethics Committee
Phytochemical screening
The plant may be considered as biosynthetic laboratory for the chemical compounds such as carbohydrates, protein, lipids, alkaloids, glycosides, tannins, etc. The compounds that are responsible for therapeutic effect are usually the secondary metabolites. A systematic study of a crude drug embraces thorough consideration of both primary and secondary metabolites derived as result of plant metabolism. The plant material may be subjected to preliminary phytochemical screening for detection of various plant constituents.
Standard screening test of the extract was carried out for various plant constituents. The crude extract was screened for the presence or absence of secondary metabolites such as alkaloids, carbohydrate, phenolic compounds, flavonoids, saponins, steroids, tannins, etc. by using standard procedures. [9][10]
Acute toxicity test
Acute oral toxicity study for the test extract of the plant was carried out using OECD/OCED guideline 425. The test procedure minimizes the number of animals required to estimate the oral acute toxicity. The test also allows the observation of signs of toxicity and can also be used to identify chemicals that are likely to have low toxicity.
Healthy, young adult albino Wistar rats (150-200 g) were used for this study. Animals were fasted (food but not water was withheld overnight) prior to dosing. The fasted body weight of each animal was determined, and the dose was calculated according to the body weight.
Limit test at 3000 mg/kg
The drug was administered in the dose of 3000 mg/kg body weight orally to one animal. This first test animal survived. Then, four other animals were dosed sequentially; therefore, a total of five animals were tested. Animals were observed individually at least once during the first 30 min after dosing, periodically during the first 24 h (with special attention given during the first 4 hr), and daily thereafter, for a total of 14 days. No animal died. Therefore, the LD 50 is greater than 3000 mg/kg. [11]
An investigation with 1/40 th, 1/20 th, and 1/10 th of 3000 mg/kg, i.e. 75, 150, and 300 mg was done in pre-screening. Only 300 mg/kg was found to be effective against diabetes, hence this dose was used in final screening.
Experimental Induction of Diabetes Mellitus
Animals were made diabetic by a single intraperitoneal injection of streptozotocin [12] (Sigma-Aldrich Company St. Louis, Missouri, USA) 30 mg/kg body weight, mixed in a freshly prepared citrate buffer (0.1M, pH 4.5) after an overnight fast. STZ injected animals were given 10% glucose solution for 24 h to prevent initial drug-induced hypoglycaemic mortality. Diabetes was confirmed by measuring the fasting blood glucose concentration 96 h after induction. Albino rats with a blood glucose level above 180 mg/dl were considered diabetic and were used in the experiment.
Experimental Design and Study Groups
After a week of acclimatization, albino rats with moderate hyperglycemia (serum glucose between 180mg/dl to 280mg/dl) were randomly divided into three diabetic groups of six rats in each while the 6 normal healthy citrate buffer treated rats served as a normal control. The groups were as under:
Group A: Normal control given normal saline at the dose – 5ml/kg body weight/day orally.
Group B: Diabetic control given normal saline at the dose – 5ml/kg body weight/day orally.
Group C: Diabetic rat treated with EEHA at the dose – 300 mg/kg body weight/day orally.
Group D: Diabetic rat treated with Glibenclamide at the dose – 5 mg/kg body weight/ day orally.
Rats were given different treatments orally once daily for 28 days. Administration was effected in a volume of 5ml normal saline/kg body weight.
Determination of Blood Sugar Level
Determination of blood glucose level both fasting (12 hour) & Post-prandial (PP) (after 2hr of food intake) were done weekly from zero to four weeks in all groups. During the period of fasting, water was given ad lib. Blood samples were drawn from tip of the tail and blood glucose levels were estimated using Accuchek Active TM glucose strips in Accu-chek Active TM Test Meter.
Statistical Analysis
Statistical analysis of the data was carried out by employing analysis of variance. For inter group comparison one way ANOVA was followed on SPSS package. The data was tested at 5% and 1% level of significance. In order to find out which of the two mean values are different from each other, the critical difference (C.D.) was calculated at 0.01 and 0.05 level. If the difference between any two means was greater than calculated critical difference (C.D.), they were considered significantly different from each other.
Results
Acute toxicity studies
A preliminary toxicity study was designed to demonstrate the appropriate safe dose range that could be used for subsequent experiments rather than to provide complete toxicity data on the extract. Acute toxicity studies conducted revealed that the administration of ethanolic extract (up to a dose of 3000 mg/kg) of H.A did not produce significant changes in behavior of the animals. No death was observed up to the dose of 3000 mg/kg b.w. The rats were physically active. These effects were observed during the experimental period (14 days). The results showed that in single dose the plant extract had no adverse effect, indicating that the medium lethal dose (LD 50) could be greater than 3000 mg/kg body weight in rats. In acute toxicity study, no toxic symptoms were observed for HA up to dose of 3 g/kg body weight. All animals behaved normally. No neurological or behavioral effects could be noted. No mortality was found up to 14 days study.
Blood glucose level
Group treated with EEHA (Group C) showed 11.37%, 21.47%, 25.33%, 32.59% decline in fasting blood glucose level & 13.85%, 17.85%, 20.65%, 34.68% decline in PP blood glucose level on 7th,14th, 21st, & 28th day respectively, which is highly significant (p<0.01) when compared with “0” day. (Tables 1 and 2)(Figure 1 and 2) Therefore this study support the earlier finding of Gopal & Chauhan (1994) claiming H.A as a novel herbal antidiabetic drug. [13]
Group treated with glibenclamide showed 22.42%, 30.64%, %, 35.8741.70% decline in fasting blood glucose level & 21.47%, 24.64%, 34.66%, 42.69% decline in PP blood glucose level on 7th,14th, 21st, & 28th day respectively, which is highly significant (p<0.01) when compared with “0” day. (Tables 1 and 2) (Figure 1 and 2)
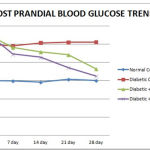 |
Figure 1: Fasting blood glucose level trend in various groups. |
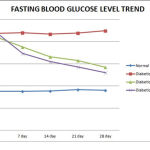 |
Figure 2: Post prandial blood glucose level trend in various groups. |
Table 1: Reduction In Fasting Blood Glucose Level In Various Groups
| FASTING | 0 day | 7 day | 14 day | 21 day | 28 day |
| Normal Control (Gr A) | 88.00 ± 3.83 | 88.17 ± 3.46 | 88.83 ± 3.17 | 91.33 ± 3.83 | 90.00 ± 3.67 |
| Diabetic Control (Gr B) | 218.67 ± 4.35 | 220.00 ± 6.02 | 216.83 ± 4.33 | 218.83 ± 6.58 | 224.17 ± 3.94 |
| Diabetic + H.A (Gr C) | 211.17 ± 8.34a | 187.67±9.16ab | 165.83 ±9.45bc | 157.67 ± 8.97c | 142.33 ±7.87c |
| Diabetic+Glibenclamide (Gr D) | 223.00± 2.78a | 173.17± 1.17b | 154.67± 1.45c | 143.00± 1.46d | 130.00±0.58e |
Values are mean ± SEM; n = 6 in each group
Values having the same superscript in row did not differed significantly.
H.A – Holarrhena antidysenterica
Table 2: Reduction In Post Prandial Blood Glucose Level In Various Groups
| POST PRANDIAL | 0 day | 7 day | 14 day | 21 day | 28 day |
| Normal Control (Gr A) | 150.0 ± 17.07 | 149.83 ±14.69 | 146 ± 18.31 | 153.5 ± 17.87 | 150.67±14.58 |
| Diabetic Control (Gr B) | 249.5 ± 12.91 | 245 ± 20.21 | 253.5 ± 18.16 | 254.83 ± 31.88 | 255.5 ± 15.4 |
| Diabetic + H.A (Gr C) | 279.17±39.34a | 240.5 ± 34.1ab | 229.33 ±32.85b | 221.5 ± 31.72bc | 182.33±25.65c |
| Diabetic+Glibenclamide (Gr D) | 284.17 ± 7.83a | 223.17 ± 4.26b | 214.17 ± 2.86c | 185.67 ± 2.58d | 162.83 ± 2.32e |
Values are mean ± SEM; n = 6 in each group
Values having the same superscript in row did not differed significantly.
H.A – Holarrhena antidysenterica
Changes in Body Weight
Diabetic rats showed an 18.42% decrease in body weight at the end of 4th week. EEHA and glibenclamide treated diabetic group rat showed significant increase in body weight Percentage increase in body weight in these (EEHA 300mg/kg b.w and glibenclamide) treated group were respectively 10.76% and 18.27% at the end of 4th week. (Table 3 and Figure 3)
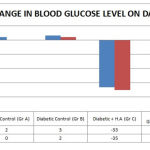 |
Figure 3 : Percentage change in body wt on 28th day |
Table 3: Reduction In Body Weight In Various Groups
| Body weight of different group (gm) | |||||
| 0 day | 7 day | 14 day | 21day | 28day | |
| Normal control | 199.2 ± 3.65 | 215.2 ± 2.7 | 221.8 ± 3.06 | 230.2 ± 2.37 | 241.3 ± 3.04 |
| Diabetic control | 190.21 ± 6.21 | 186.4 ± 6.01 | 173.6 ± 5.82 | 160.4 ±5.25 | 155.4±3.25 |
| Diabetic rats + H.A (300mg/kg) | 195.6 ± 5.16* | 205.6 ± 4.95** | 210.4 ± 5.25** | 213.2 ± 2.27** | 216.2 ± 1.45** |
| Diabetic rats + glibenclamide (5mg/kg) | 186.1 ± 5.89* | 193.8 ± 5.76** | 202.4 ± 6.48** | 212.6 ± 3.17** | 220.6 ± 2.67** |
Values are mean ± SEM; n = 6 in each group
*P > 0.05 (non-significant) **P < 0.01 (highly significant) when compared to normal control and diabetic control rats.
Discussion
The various numbers of plants have been traditionally used to treat diabetes, and some have been proven to have hypoglycemic effects. These studies have identified that compounds such as polysaccharides, flavonoids, terpenoids and tannins, and steroids are responsible for antidiabetic effect [14]. EEHA also contains flavonoids, saponins and carbohydrate, steroids, tannins, and phenolic compounds. The observed hypoglycemic effects of this plant could have resulted from the combined activity of these compounds present in the extract.
Administration of STZ caused rapid destruction of pancreatic β-cells in rats, which led to impaired glucose stimulated insulin release and insulin resistance, both of which are marked feature of type II diabetes. [15] Present drugs which are used for the treatment of this disease are mainly insulin, sulphonylureas and biguanides. All these drugs are associated with adverse effect and not able to control metabolism adequately. Management of diabetes with agents devoid of any side effects is still a challenge to the medical system. There is growing interest in herbal remedies due to these reasons. [16] The present investigation indicates the hypoglycemic effects of EEHA on STZ-diabetic rats. We have observed a significant (P < 0.01) decrease in blood glucose in EEHA-treated diabetic rats, when compared with diabetic control rats. Similar results of the methanolic extract of this plant seeds were reported by mana et al. [17] The possible mechanism of EEHA on hypoglycemic action may be through potentiation of pancreatic secretion of insulin from b-cell of islets and/or due to enhanced transport of blood glucose to the peripheral tissue or by other mechanisms such as stimulation of glucose uptake by peripheral tissue, inhibition of endogenous glucose production or activation of gluconeogenesis in liver and muscles. [18]
Decrease in body weight in streptozotocin induced diabetic rats and weight gain in EEHA treated group is due to loss of tissue protein [19] and muscle wasting in the former and having beneficial effect in preventing loss of body weight and in catabolic process in EEHA treated groups. Significant increase in the weight of the animals treated with EEHA in comparison to vehicle treated diabetic rats indicating that ethanolic extract had beneficial effect in preventing loss of body weight of diabetic rats.
Conclusion
This observations confirm that ethanolic extract of seeds of the plant has antidiabetic activity and is also involved in preventing loss of body weight. It also warrants further investigation to isolate and identify the hypoglycemic principles in this plant so as to elucidate their mode of action.
References
- IDF Diabetes Atlas, 4th edition. International Diabetes Federation,2009 S. Wild et al; diabetes care 27; 1047, 2004
- Gupta Soumya, Mal Mainak, Bhattacharya Plaban, European Bulletin of Drug Research 2005,13,5155.
- Neeraj Kumar, Steroidal Alkaloids from Holarrhena antidysenterica Pharm. Bull. 55(6) 912—914 (2007)
- Dutta, N.K., and Iyer, S. Natrajan (1968) antiamoebic value of burberine and kurci aldaloids, journal of the Indian medical association. 50(8)349-52
- Chopra, R. N., Gupta J.C., David, J.C.and Ghosh S. (1927). Observation on the pharmacological action of conessine the alkaloids of H.antidysenterica Indian med gaz. 68:132
- Dhar ML, Dhar MM, Dhawan BN, Mehrotra BN, Ray C. Screening of Indian plants for biological activity: I. Indian J Exp Biol 1968;6(4):232-247.
- Dama GY, Rao MEB. Anti-diabetic activity of holorrhena antidysentrica (linn) wall, Bark on streptozotocin induced diabetic rats. International journal of intuitional pharmacy and life sciences 2011; 1(2):24-38.
- Subash P., Prabuseenivasan S. and Ignacimuthu S., Phytomedicine, 2007, 14, 15-22.
- Khandelwal KR. Practical Pharmacognosy techniques and experiments. 14 th ed. Pune: Nirali Prakashan; 2005. p. 150-3
- Kokate CK. Practical Pharmacognosy. 4 th ed. Delhi: Vallabh Prakashan; 1997. p. 108-11
- OECD guidelines for the testing of chemicals (Acute oral toxicity – up and down procedure). Cited 2008 Mar 20]; Available from: http://www.oecd.org
- T. Szkudelski The Mechanism of Alloxan and Streptozotocin Action in beta Cells of the Rat Pancrease Physiol. Res. 50: 536-546, 2001
- Gopal, V. and Chauhan, M.G ;( 1994) Holarrhrena antidysenterica a nobel herbal antidiabetic drug. 45th IndianPharm. Cong. New Delhi held on Dec.23-25, 1993 in Indian Journal of Pharmaceutical Basis of Therapeutics.9th ed pp1493-1498. McGraw Hill Companies, Inc., United States of America
- Sabu MS, Smitha K, Ramadasan K. Anti-diabetic activity of green tea polyphenols and their role in reducing oxidative stress in experimental diabetes. J Ethnopharmacol 2002;83:109-16
- Elsner M, Guldbakke B, Tiedge M, Munday R, Lenzen S. Relative importance of transport and alkylation for pancreatic beta-cell toxicity of streptozotocin. Diabetologia 2000;43:1528-33
- Pradeepa R, Mohan V. The changing scenario of the diabetes epidemic: implication for India. Indian J Med Res 2002; 116:121-32.
- Mana S, Singhal S,Sharma NK,Singh D. Hypoglycemic Effect of Holarrhena antidysenterica Seeds on Streptozotocin induced Diabetic Rats. International Journal of PharmTech Research 2010;2(2):1325-1329
- Burcelin R, Eddouks M, Maury J, Kande J, Assan R, Girard J. Excessive glucose production rather than insulin resistance accounts for hypoglycemia in recent onset diabetic rats. Diabetologia 1995;38:283-290
- Chatterjea MN, Shinde R. Diabetes mellitus. In: Text Book of Medical Biochemistry. New Delhi: Jaypee Brother Medical Publisher; 2002.p. 317








