Manuscript accepted on :
Published online on: 25-11-2015
Plagiarism Check: Yes
N. Vigneswaran1, Fahmi Samsuri1 and Nithya Kalyani.K2
1Faculty of Electrical and Electronics Engineering, Universiti Malaysia Pahang, Pekan 26600, Malaysia 2Department of Biomedical Engineering, Vel Tech multitech Dr.RR and Dr.SR Engg college.Chennai,India
DOI : https://dx.doi.org/10.13005/bpj/618
Abstract
In this article we report the development of scaffold mimicking the cell surface topography. Using Soft lithography, in which polymer (triglyme, ethylene glycol dimethacrylate, methacrylic acid and IRGAcure 2022) is applied over the cultured cell and cured instantly using UV source. IRGA cure 2022 is a photoinitiator, which helps in solidification of the polymer when exposed to UV light source. Triglyme helps to increase the viscosity of the polymer. This method can be applied to all adherent cells. This scaffold consists of cell surface topography features. The developed scaffold can pave way in tissue engineering for various applications and cell research investigations.
Keywords
cell; morphology; feature; scaffold; extracellular matrix and tissue engineering
Download this article as:| Copy the following to cite this article: Vigneswaran N, Samsuri F, Kalyani. K N. Bioimprinting Based Scaffold Development for Tissue Engineering Applications |
| Copy the following to cite this URL: Vigneswaran N, Samsuri F, Kalyani. K N. Bioimprinting Based Scaffold Development for Tissue Engineering Applications. Biomed Pharmacol J 2015;8(1). Available from: http://biomedpharmajournal.org/?p=1605 |
Introduction
Knowledge on combining cells from the body with highly porous scaffold biomaterials plays a vital role in tissue engineering applications such as cell culture using scaffold and regeneration of damaged tissues. These biomaterials can act as templates for tissue regeneration. The field of Tissue Engineering is currently limited in the range of biomaterials available for development of tissue engineered products. In addition to the synthesis of scaffold biomaterials, a material which has mechanical properties as the benchmark for the cell and tissue organisation has attracted interest of scientific research and philosophical notions. Relevant perusal of developing more sophisticated biomimetic biomaterials with added levels of Extracellular matrix and to encourage the biomaterials to guide cell organisation is still a state-of-the-art interest consistent with the conventional techniques.
Most of the preclinical drugs, in all therapeutic classes, fail to result in efficacious human treatments. This leads to wasting vast amounts of time and money and which in turn delays the discovery of successful interventions.[1] Tissue culture in glass slide models lack realistic complexity, while animal models are expensive, time consuming, and too frequently fail to mimic human tumor biology.[2]
Study of biochemistry of cells, drug screening and testing of toxicity can lead to seed the cells either in flask or by means of the scaffold. Tissue engineering has evolved out of the need to cell organisation. A tissue-engineering scaffold is a critical component of a clinically successful and commercially available system used for both guiding the cell organisation and the surgical replacement of lost skin. Another tissue engineering pursuit refers to design a scaffold material for bone tissues. This may improve the standard for the treatment of bone defects. Scaffolds are an integral part of bone tissue engineering. Scaffolds are three dimensional (3D) biocompatible structures which can mimic the Extracellular matrix properties, and provide a template for cell attachment and stimulate bone tissue formation in vivo.
The ideal properties of the scaffolds are as follows,
- It should have radical scavenging ability.
- Growth factor delivery.
- Replace lost cells/tissues.
- Mimics native extracellular matrix.
- It should promote cell adhesion, Viability and proliferation.
- It should provide better contact guidance
The basic sources for the manufacturing of scaffold materials are bovine sources, Sea shells and the polymer based materials. The commonly used materials are polymer based, chitosan derived from chitin and collagen fiber. The collagen acts as a protein element in extracellular matrix (ECM) which may grow cells and it also has cell adhesive property. The most promising application of the scaffold is that it can be used as the culture medium for the cell culture for the cell study. Cell culture has become one of the major tools in the life sciences today. This study serves as the guidance for the cell culture and this may be the pioneering study for using an alternate medium for the cell culture. Some of the important areas where cell culture is currently playing a major role are mentioned below,
- It can be used in the Cancer research. The cultured cancer cells also serves as a test system to determine suitable drugs and methods for selectively destroying types of cancer cells
- One of the major uses of cell culture is the replication of viruses for use in the vaccine preparation.
- It can be used in cell- drug study and investigations.
This paper is organized as follows: Section 2 elaborates various Scaffolding approaches and scaffolds used for the various tissue engineering applications and different materials used will also be discussed. In Section 3 and 4, the proposed imprint technique for designing the Scaffold is presented and the results obtained were discussed. In Section 5 and 6, concluding remarks and applications are furnished.
Various Scaffolding Approaches
The cell, scaffold and growth factor are the three key materials for tissue engineering. The cell synthesizes matrices of new tissue, while the scaffold provides the appropriate environment for cells to be able to effectively accomplish their missions. The function of growth factors is to facilitate and promote cells to regenerate new tissue.[3, 4] Significant extent of use of scaffolds in the tissue engineering applications has been carried out in mid 1980’s. The vast majority of the studies refer to the four basic scaffolding approaches are available.[5] It includes the approaches such as Pre-made porous scaffolds for cell seeding, Decellularized ECM from allogenic or xenogenic tissues for cell seeding, Cell sheets with self-secreted ECM and Cell encapsulation in self-assembled hydrogel matrix. Each approach has its own pros and cons and preferred tissue engineering applications. In planning for tissue engineering for a complex tissue such as Intravertebral discs (IVD), these scaffolding approaches serves as important guidelines and can be used in combinations. The methods are summarized in Fig. 1.
The fabrication of scaffold to mimic the real tissue is a challenging task in in-vitro tissue engineering applications. The scaffold design achieves changes in cell orientation was carried out by designing models consists of wells with different pattern density material.[6] Various scaffolds are developed for tissue engineering applications.[7]
Scaffold used for the cell culture applications
Cell culture or the tissue culture is the general term used for the removal of cells from animal or the human and placed these cells in the artificial environment for the considerable growth.
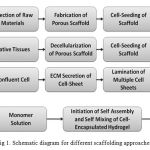 |
Figure 1: Schematic diagram for different scaffolding approaches |
Culture conditions vary widely for each cell type, but the artificial environment in which the cells are cultured invariably consists of a suitable vessel containing a substrate or medium that supplies the essential nutrients, growth factors, hormones, and gases, and regulates the physicochemical environment. There are two primary culture systems for the growth of cells, one is Monolayer culture (adherent culture) system and the other one is Suspension culture system. The ability of the cells to grow attached to a glass or a treated plastic was discussed in the Monolayer culture system and other type discussed about floating free in culture medium. Cells that are cultured in suspension can be maintained in culture flasks that are not tissue-culture treated.
Culture medium
The culture medium is the most important component of the culture environment, because it provides the necessary nutrients, growth factors, and hormones for cell growth, as well as regulating the pH and the osmotic pressure of the culture. The three basic classes of media are
- Basal media,
- Reduced serum free media and
- Serum – free media
Serum media plays a vital role as the source for a growth of cells, adhesion factors, lipids and minerals for culturing cells. However it was an essential source, it has some disadvantages such as high cost and problems with standardization, variability and inhibition of growth on certain cell culture. The basal media contains the amino acids, vitamins and inorganic salts but these media also provided with some supplement function of the serum media. The serum free media can be advised as the proper media for the cell culture but it also has its own disadvantages that it needs for higher degree of reagent purity and the slower growth of cells was observed in the serum free media. All these disadvantages lead to pioneering study of developing scaffold as the medium for the cell culture. The cells grown in a scaffold possess a natural tissue like structure.
Scaffolds as a culture medium
In conventional 2D cell culture, cells come into contact with the flat surface of the culture vessel (e.g. Petri dish, flask or multi-well plate). In this unnatural environment cells become flattened against the substrate. The 3D scaffold (Alvetex) method was proposed to culture a cell which possess nature cell like structures and shapes.
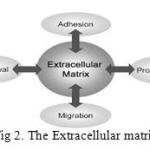 |
Figure 2: The Extracellular matrix |
The natural cell growth is mainly due to the Extracellular matrix present in the cell mutation. The ECM is a collection of extracellular molecules secreted by cells that provides structural and biochemical support to the surrounding cells. It is a substance containing collagen, elastin, proteoglycans, glycosaminoglycans, and fluid, produced by cells and in which the cells are embedded. The matrix secreted by chondroblasts, for example, is responsible for the properties of cartilage. Fig. 2 represents the various factors and functions of extracellular matrix.
The ECM is responsible for the cell growth and the cells should interact with the specific components of the ECM. It is a network of proteins and carbohydrates that binds the cells together and it regulates cell activities. It behaves as a lattice for the cell movement. The functions of ECM are to provide a mechanical support, embryonic development, pathways for the cell migration.[8-11] The 3D biomaterial scaffold can more closely replicate the cells in the nature environment. Natural biomaterials are often composed of proteins and polysaccharides found in the extracellular matrix and as a result contain binding sites for cell adhesion and readily support cell culture Some natural biomaterials such as fibrin provides a structure similar to the natural ECM and so the cells cultured in the scaffolds assesses the cell growth like natural cell when compared to the cells cultured in the flasks and some artificial environment.
The fibrin scaffolds can be used as the culturing medium for the stem cells. As discussed in the study of fibrin scaffolding approaches [12], the fibrin scaffolds from fibrinogen solutions using the enzymatic activity of thrombin was widely used in the stem cell culture applications. A method [13] has been reported to develop a scaffold used for the cell culture. In this method, the authors developed an alternative cell culture medium composed of autologous fibrin, due to their low fibrinogen concentrations it can be used for the suitable cell growth. The cells cultured in the flasks may be contaminated by the virus and the fungus like microorganisms. These kinds of contaminations can be avoided in the scaffold medium. The scaffold offers a happy and contamination free environment for the cell growth as occurs in the Extracellular matrix. As discussed earlier the cell culture has been applied in many fields such as cancer research, Virology and the toxicity testing. It can also be used for the study of biochemistry of cells, drug screening. The major advantage of using cell culture for any of these applications is the consistency and reproducibility of results that can be obtained from using a batch of clonal cells.
Scaffold fabrication by lithography
New method integrating nanoimprint lithography directly with biological materials to create replica cell impressions in a robust storage medium has been developed to facilitate topographic analysis using nano-imaging tools termed Bioimprint. Earlier studies using a heat curable polydimethylsiloxane or a UV-curable elastomer was introduced for replication to facilitate cell imaging. Bioimprinting of human nasal chondrocyte [14] have been carried out. The scaffold which can be used to culture the medium should possess the key factors that cell adhesion, proliferation and cell organisation. Various techniques have been adopted in order to mimic the ECM in the scaffold such as electron beam lithography and spin on nanoprinting (SNAP).[15] An imprinting technique can be utilized to facilitate the cell adhesion and alignment.
The adopted study is about the development of scaffold material which can be used for the tissue engineering applications
Methods
Cells were cultured by standard culturing methods. Cell lines used in this are endometrial Ishikawa cancer cells, but any adherent cells can also be used. Glassware and equipments used for cell preparation was autoclaved and sterilized with 70% ethanol before use. Cells were grown in 1000 mL of α-MEM which was supplemented with NaHCO3 (2.2 g), penicillin/streptomycin (10 mL, Gibco 15070-063), fungizone (4 mL) and fetal bovine serum (100 mL, Gibco 10093-144). Cultures are placed at 37°C, in an environment of 0.5% CO2.
Cell growths were monitored by microscope. Once cells attained the confluent state, it is then coated with polymer composed of 1.2 mL of triglyme, 0.425 mL of ethylene glycol dimethacrylate, 0.0425 mL of methacrylic acid and 20 µL of IRGAcure 2022 (CIBA Specialty Chemicals). UV curing was done using UV lamp (100WHg arc lamp, 20% iris setting, and 250–450 nm filter). After UV curing, the polymers are cross linked. Solidified polymer is peeled off leaving the cell topography features imprinted on the polymer. Then it is rinsed and washed to remove any residuals. Fig. 3 clearly explains the development process of the scaffold.
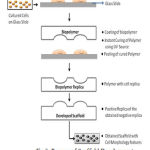 |
Figure 3: Process of Scaffold Development |
Results and Discussion
Cells were cultured and constantly observed through microscope for confluent state. This helps in transferring the features much better with clear imprinting by reducing the overlapping of the cells. Developed scaffold consists of micro morphology features of cell. Fig.4 shows the 2D and 3D AFM image of the developed scaffold with micro cell surface features respectively. Imaging of the obtained Scaffold was done using Digital Instruments (DI) 3100 Nanoscope III AFM in tapping mode. Images are scanned at the rate of 0.3007Hz. Scan size of the images are 100µm. Surface topographies are clearly visible in the image.
 |
Figure 4: A) 2D AFM image of Scaffold developed and B) 3D AFM image of Scaffold developed |
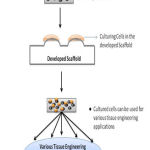 |
Figure 5: Applications of Developed Scaffold |
Conclusion
Simple and easy method has been used to develop the scaffold for tissue engineering. Polymer based scaffolds have some disadvantages such as inflammation during the implantation, which needs to be taken care for specific applications. In vitro cell investigations can be facilitated by the developed scaffold. Further a positive replica of obtained imprint can be made, for cell research investigations.
Applications
The developed scaffold can find its way in all kinds of tissue engineering applications. Fig. 5 shows the application of the scaffold developed. Originality of the cells are maintained by mimicking the morphology features in scaffold. It can be used in all kinds of cell research investigations which involves culturing of cells.
Acknowledgment
Authors would like to thank and acknowledge for the support and fund provided by University Malaysia Pahang.
References
- van der Worp, H.B., et al., Can animal models of disease reliably inform human studies? PLoS medicine, 2010. 7(3): p. e1000245.
- Aggarwal, B.B., et al., Models for prevention and treatment of cancer: problems vs promises. Biochemical pharmacology, 2009. 78(9): p. 1083-1094.
- Dado, D. and S. Levenberg, Cell–scaffold mechanical interplay within engineered tissue. Seminars in Cell and Developmental Biology, 2009. 20(6): p. 656-664.
- Anselme, K., A. Ponche, and M. Bigerelle, Relative influence of surface topography and surface chemistry on cell response to bone implant materials. Part 2: biological aspects. Proceedings of the Institution of Mechanical Engineers, Part H: Journal of Engineering in Medicine, 2010. 224(12): p. 1487-1507.
- Chan, B. and K. Leong, Scaffolding in tissue engineering: general approaches and tissue-specific considerations. European spine journal, 2008. 17(4): p. 467-479B.
- Senderling, B., et al. Design of a microfeature scaffold for tissue engineering. in Bioengineering Conference (NEBEC), 2011 IEEE 37th Annual Northeast. 2011. IEEE.
- Karp, J.M., P.D. Dalton, and M.S. Shoichet, Scaffolds for tissue engineering. MRS bulletin, 2003. 28(04): p. 301-306.
- Hay, E.D., Cell biology of extracellular matrix. 1991: Springer.
- Davies, J.A., Extracellular matrix. eLS, 2001.
- Frantz, C., K.M. Stewart, and V.M. Weaver, The extracellular matrix at a glance. Journal of cell science, 2010. 123(24): p. 4195-4200.
- Lehnert, D., et al., Cell behaviour on micropatterned substrata: limits of extracellular matrix geometry for spreading and adhesion. Journal of cell science, 2004. 117(1): p. 41-52
- Ko, J., et al., Towards high throughput tissue engineering: development of chitosan-calcium phosphate scaffolds for engineering bone tissue from embryonic stem cells. American journal of stem cells, 2012. 1(1): p. 81.
- de la Puente, P. and D. Ludeña, Cell culture in autologous fibrin scaffolds for applications in tissue engineering. Experimental cell research, 2014. 322(1): p. 1-11.
- Nock, L. Murray, F. Samsuri, M. M. Alkaisiand J. J. Evans,”Microfluidics-assisted photo nanoimprint lithography for the formation of cellular bioimprints”,J. Vac. Sci. Technol. B 28, C6K17 (2010); http://dx.doi.org/10.1116/1.3501342.
- Duong, B. and M. Su, Imprinted nanotextured scaffolds for cell guiding and migration.







