Reza Ranjbar1, Mehdi Moazzami Goudarzi2٭, Nematollah Jounaidi3
1Molecular biology research center, Baqiyatallah University of medical science, Tehran, Iran. 2Department of Microbiology, Boroujerd Branch, Islamic Azad University, Boroujerd, Iran. 3Health Research Center, Baqiyatallah University of medical science, Tehran, Iran Corresponding author Email: Moma1675@gmail.com
Abstract
The present study aims to evaluate the effects of lactobacillus by-products on pyelonephritis-associated pili pap expression in Escherichia (E.) coli. Vaginal lactobacillus rhamnosus and lactobacillus crispatus species were isolated and the effects of their culture supernatants (CS) on pap promoter activity in uropathogenic E. coli (UPEC) were studied. A new reporter construct of pET28a including Iranian firefly luciferase coding sequence was used to assess the pap propotor activity. The lactobacillus rhamnosus forced the pap promoter to turn off when UPEC was exposed to 1%, 1.1%, and 1.3% of the culture supernatant. In addition, lactobacillus crispatus could continuously switch off pap promoter phase variations when UPEC was exposed to a serial dilution of culture supernatant. The findings showed therapeutic and preventive potential of lactobacillus rhamnosus and lactobacillus crispatus for bacterial vaginitis and urinary tract infection (UTI) through controlling the virulence factors expression via their ecological origins.
Keywords
Escherichia coli; Vaginitis; Urinary Tract Infection; lactobacillus rhamnosus; lactobacillus crispatus
Download this article as:| Copy the following to cite this article: Ranjbar R, Goudarzi M. M, Jounaidi N. Vaginal Lactobacilli and Pap Operon Expression in Uropathogenic Escherichia Coli. Biomed Pharmacol J 2015;8(March Spl Edition) |
| Copy the following to cite this URL: Ranjbar R, Goudarzi M. M٭, Jounaidi N. Vaginal Lactobacilli and Pap Operon Expression in Uropathogenic Escherichia Coli. Biomed Pharmacol J 2015;8(March Spl Edition). Available from: http://biomedpharmajournal.org/?p=2346> |
Introduction
Escherichia (E.) coli is the most commonly isolated organism in urinary tract causing >70% of uncomplicated and 25-50% of complicated urinary tract infections (UTIs) [1]. E. coli causes vaginal infections such as aerobic vaginitis, can is found in vaginal microbiota of up to 20% of women during their lifespan [2]. This implies the presence of an asymptomatic E. coli reservoir in a majority population of women as well as its important role in preventing development of urogenital tract infections in local vaginal environment. The high prevalence of E. coli in vaginal colonization and subsequent UTI is primarily due to their large numbers constantly shedding in feces and promoting frequent urogenital contacts.
coli inherently exhibits different attributes that make it survives under varying environmental conditions including short generation time and ability to metabolize a wide variety of carbon sources and ability to perform facultative anaerobic metabolism. However, only a few numbers of strains can successfully survive, colonize, and cause infection within the urogenital tract. A possible sequel includes pyelonephritis, which can lead to renal scarring and sepsis [3, 4]. Uropathogenic E. coli (UPEC) exhibits a set of specific virulence factors (VFs) involved in host cell attachment and invasion, biofilm formation, host cell cytotoxicity, iron acquisition, evasion of host defenses, and increased antibiotic resistance [5]. A number of virulence determinants enhance the ability of UPEC to colonize the urinary tract and exert cytopathic effects, including type 1 fimbriae [6], P fimbriae [7], adhesions [8], hemolysin [9, 10], cytotoxic necrotizing factor 1, [11], flagella [12], capsule polysaccharide [13], lipopolysaccharide O antigen [14], and TonB-dependent iron transport system [15]. During UTI, the outer membrane proteins of UPEC like porins (OmpA, OmpC, OmpX, NmpC, and LamB) and outer membrane assembly factors are overexpressed [16].
More than 68% of Iranian healthy adult women and less than 10% of them are colonized by lactobacillus rhamnosus and lactobacillus crispatus separately during intimate contacts. Moreover, these two lactobacilli contribute to the formations of most vaginal microflora in women with lower chance of recurrent UTI [7]. Therefore, it is necessary to conduct a comparative study to clarify the roles of by-products of lactobacillus rhamnosus and lactobacillus crispatus in the prevalence of UPEC colonization in the urinary tract. The present study evaluates the effects of two potential probiotic strains of lactobacilli and their by-products on UPEC growth and expression of VFs d. Attachment of pyelonephritis-associated pili (PAP)is critical for UPEC colonization in upper urinary tract; therefore, `cted adherent Pap operon for study. Our study aimed at investigating how lactobacilli by-products can affect pap fimbrial operon expression separately.
Fimbrial operons include PAP operons with 7-8 structural genes responsible for constructing Pap [17, 18]. P pili are associated with E. coli strains, capable of directly infecting upper urinary tract tissues [19]. PAP operons are controlled by a phase variation mechanism, in which individual bacterium could express (ON) or repress (OFF) fimbriae [19]. Phase variation in these operons is controlled at the transcriptional level by a complex epigenetic mechanism involving the formation of specific DNA methylation patterns similar to certain eukaryotic systems [20]. UPEC survival under environmental stresses such as increased acidity involves a number of factors aiming at maintaining membrane integrity and cytoplasm neutrality. Outer membrane proteins play a significant role in these processes by controlling the movement of compounds across the outer membranes [21, 22].
Materials and Methods
Study Requirements
In first step, more than 50 vaginal specimens were collected form adult healthy women. SMEL criteria were used to categorize the women into two healthy and patient groups. The healthy women were selected for lactobacillus preparation. Specimens were cultured in Man, Rogosa, and Sharpe (MRS) agar medium and then characterized by macroscopic and microscopic features through biochemical and molecular assessments to isolate Lactobacillus rhamnosuss and lactobacillus crispatus. UPEC isolate from a patient suffering from pyelonephritis was used as a wild-type UPEC. In addition, recombinant pET28a cloning vector containing lampyris turkestanicus (Iranian firefly) luciferase coding sequence (GenBank accession No. AY742225.1) was used. This cloning vector imposes restriction sites for BamHI and HindIII, flanking the luciferase coding sequence.
Lactobacillus Characterization
Because of the specific sugar fermentation patterns in lactobacilli, a set of sugar fermentation tests including Maltose, lactose, mannose, arabinose, fructose, cellobiose, melibiose, raffinose, rhamnose, sorbitol, sucrose, xylose, and trehalose were performed separately for the isolated wild-type and standard strains. For confirmation test, 16s rDNA amplification was conducted with the following specific primers:
(Lacto) F: 5´- TGGAAACAGTGCTAATACCG – 3´ and R: 5´- TCCATTGTGGAAGATTCCC – 3´ for genus confirmation; (rhamno) F: 5´- TGCTTGCATCTTGATTTAATTTTG – 3´ and R: 5´- GGTTCTTGGATTATGCGGTATTAG – 3´ for lactobacillus rhamnosus confirmation; and finally (Crispa) F: 5´- TTACTTCGGTAATGACGTTA – 3´ and R: 5´- GGAACTTTGTATCTCTACAA – 3´ for lactobacillus crispatus confirmation.
Amplification of PAP Promoters
First, pap regulatory region (406 bp) was amplified by the following primers: primer PAPF1 containing BglII recognition site (PAP F1: 5´-TCAGATCTTCATCATCTCACTG-3´), and PAPR1 (PAP R1: 5´-CATGGATCCCCCTTCTGTCGGG-3) carrying BamHI recognition site underwent PCR with the fallowing conditions: Pre-denaturation at 94 °C for 3 min, denaturation at 94°C for 1 min, annealing at 57 °C for 1 min, and extension at 72 °C for 1 min. This procedure was repeated for 35 cycles. Final extension was performed at 72°C for 3 min. Afterwards, OmpA promoter was amplified using OmpAF1 and OmpAR1 primers containing BglII and BamHI recognition sites. PCR was implemented under the same conditions with the exception of annealing temperature that was adjusted at 59 °C.
Construction of Recombinant Vectors
First, pET28a vector carrying luciferase coding sequence was double-digested with BamHI and BglII. Then, PAP-PCR product was double-digested with the same restriction enzymes. Both the vector and PCR products were ligated together to construct luciferase-controlled PAP on the new vector pETpap28a. The ligation mixture contained the following elements: 2 µl of double-digested vector, 10 µl of double-digested PAP regulatory region of PCR product, 1 µl of T4 DNA ligase, 5 µl of dd water, and 2 µl of T4 ligase buffer with a final volume of 20 µl at 16°C for almost 17h. After construction, recombinant vector was transformed into UPEC isolate to prepare light emitting reporter strains separately. The reporter construct only consisted of the regulatory region lacking any structural and regulatory genes in pET28a. Therefore, the expression of PAP-luciferase construct was directly regulated by chromosomal regulatory proteins.
Bacterial Growth
The transformed bacteria were analyzed for growth and promoter activity during 24 hours of exposure to lactobacillus culture supernatant (Cs) in modified M9 (MM9) medium. To reach the standard growth conditions, the bacteria (UPECs) were cultured in 100ml Erlenmeyer containing 10 ml of M9 glycerol (M9 minimal medium consisting of 30 mM thiamine, 100 mM calcium chloride, 1 mM magnesium sulfate, and 0.2% glycerol as carbon source, pH 7). However, to assess the effect of lactobacillus Cs, the modified M9 medium including 0.1-1.5% of lactobacillus Cs was prepared.
Catalase test was carried out to clarify whether H2O2 had been secreted into culture supernatant. Moreover, the effect of a serial dilution (0.05-0.5% of H2O2 in MM9 medium) on pap promoter activity was assessed versus culture supernatant. Meanwhile, the effect of a serial dilution of pure lactic acid (0.1-1.5%) was evaluated versus culture supernatant. All the above-mentioned bacterial growth conditions were considered for vaginal wild-type lactobacilli and standard control spices separately.
Measurement of bioluminescence activity
In this procedure, equal volumes (20 µl) of both overnight cultures of reporter strain and luciferin substrate solution were mixed in a luminometer cuvette. The emitted light intensity was measured at 530 nm according to bioluminescence unit (relative light unit per second (RLU/S)). Substrate solution was composed of (i) 4mM ATP solution, (ii) 2 mM D-luciferin, (iii) 5 mM MgSO4 solution, and (iv) 50 mM Tris-HCl. In final step, pH of substrate solution was adjusted to 7.8 by Tris-HCl. The stock solution was kept frozen at -20 °C. All the samples were evaluated for light emission every 2 hours, when they had the same optical density.
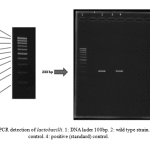 |
Figure 1: PCR detection of lactobacilli. 1: DNA lader 100bp. 2: wild type strain. 3: negative control. 4: positive (standard) control |
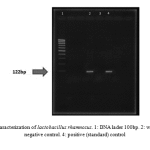 |
Figure 2: PCR characterization of lactobacillus rhamnosus. 1: DNA lader 100bp. 2: wild type strain. 3: negative control. 4: positive (standard) control |
 |
Figure 3: PCR characterization of lactobacillus crispatus . 1: DNA lader 100bp. 2: wild type strain. 3: negative control. 4: positive (standard) control |
Table 1: sugar fermentation pattern of lactobacilli
| L rhamnosus | L. crispatus
|
|
| Lactose | + | + |
| Mannose | + | + |
| Arabinose | – | + |
| Fructose | + | + |
| Maltose | + | + |
| Cellobios | – | – |
| Melibios | – | – |
| Raffinose | + | + |
| Rhamnose | + | – |
| Sorbitol | – | + |
| Sucrose | + | – |
| Xylose | + | + |
| Trehalose | – | – |
Results
Sugar fermentation pattern and molecular specification were used by the specific primers for lactobacillus characterization based on PCR test. Table 1 represents the results of sugar fermentation. There was no difference between the wild-type and standard strains. Amplification with the specific primers led to a 322 bp product for genus confirmation (Fig. 1). In addition, 122bp and 966bp PCR products were separately amplified for Lactobacillus rhamnosus and Lactobacillus crispatus (Fig. 2 & Fig. 3).
Light emission was assessed after UPEC recombinant strain treatment under the conditions described above. Lactobacillus culture supernatant used in the continuous mode was able to affect the pap promoter activity. In the case of lactobacillus rhamnosus, pap promoter activity was significantly increased to 0.1-0.3% Cs in MM9. Furthermore, the pap promoter activity decreased when exposed to 0.4-0.5% of Cs, showing an approximate stability of 0.6-1% of Cs. Afterwards, light emission was increased in MM9 medium with the exception of 1%, 1.1%, 1.3%, and 1.5% of Cs. The serial dilution of pure lactic acid in MM9 medium indicated significant decrease of light emission by an increase in lactic acid concentration. Considering H2O2 as a potent active ingredient in culture supernatant, light emission had no significant difference during exposure to 0.05-0.2% of H2O2 in the culture supernatant (1458 RLU/S). However, the pap promoter activity was decreased to 567 RLU/S when the recombinant reporter UPEC strain was influenced by MM9 medium including 0.5% of H2O2. No significant difference was found between the results of wild-type lactobacillus and the control strain.
In the case of lactobacillus crispatus, pap promoter activity was decreased continuously from 2015 RLU/S to 830 RLU/S as culture supernatant concentration was increased in MM9 medium. Exposure of the reporter strain to 1-1.5% of pure lactic acid was able to decrease light emission, finally turning it off. Considering H2O2 as a potent active ingredient in culture supernatant, light emission had at least a significant alteration during exposure to 0.05-0.2% of H2O2.
Pap promoter activity started to reduce when exposed to MM9 medium including 0.05% (1023 RLU/S) up to 0.2% (930 RLU/S) of H2O2. Interestingly, the pap promoter activity was increased by 0.3% of H2O2 in MM9 medium, resulting in a decrease in light emission. Yet, in the case of standard control strain, the pap promoter activity seemed to decrease continuously contrary to the increase in H2O2 content in MM9 medium. With the exception of H202, no other significant difference was witnessed between lactobacillus crispatus wild-type and standard strains.
Conclusion
Urogenital tract is constantly under assault by microorganisms. Usually, these microorganisms originate from the surrounding environment, especially the skin and feces. Nevertheless, from among various vaginal microbiota, only a selected number of pathogenic bacteria are able to readily colonize and cause infection [23]. Based on their presence in the vagina even in some healthy women, one would expect UPEC to play a major role in vaginal infections as well. The inhibitory effect of lactobacillus CS is presumably relates to lactic acid and hydrogen peroxide. The two potent inhibitors of UPEC growth support the protective role of lactobacillus against UPEC strains. Although lactic acid is a weak acid, it has been shown to exhibit potent antibacterial effects on numerous pathogens including UPEC, especially under nutrient-limiting conditions such as those observed in the vagina.
Our findings showed the inhibiting potential of lactobacillus CS, lactic acid, and hydrogen peroxide for UPEC Vf expression indicating the protective role of lactobacillus against UPEC strains. CS effects were likely beacuse of lactic acid since the results of both pH-balanced and -unbalanced CS mimicked those of lactic acid. Herein, we observed enhancement in concentrations not above 2% and almost 70% of growth reduction was measured to as little as 1.5%. Previous investigations have determined that 24-hour cultures of L. rhamnosuss GR-1 and L. reuteri RC-14 produce approximately 45 and 35 mM lactic acid, respectively [24]. Since the normal vaginal lactic acid content is typically measured between 10-50 mM, our results supported lactic acid play a major role in UPEC inhibition by lactobacilli within lactobacillus rhamnosus culture supernatant. Hydrogen peroxide is a potent antibacterial compound produced by many strains of lactobacilli [17] and has been shown to induce bacterial membrane stress [18]. Notably, both pH-neutralized CS and lactic acid actually stimulate growth [17]. This can create varied complications in the urogenital tract, especially when amine-producing pathogens such as Prevotellabiviaare are present [6]. As amine production raises the vaginal pH, the lactobacilli produced by lactic acid may transform from an antibacterial compound to a carbon source for the organisms like UPEC that cause bacterial vaginitis associated with Prevotella. Also, our findings may offer some explanations as to why vaginal infections sometimes occur despite lactobacillus colonization. Our unpublished data promoted the facts about pap promoter different activities when an hns mutant of UPEC is exposed to lactobacillus CS in MM9 medium. In UPEC hns mutant, pap promoter exhibited a different activity compared to a wild-type UPEC strain. P fimbriae was constructed when a UPEC hns mutant was exposed to MM9 medium including 0.4-0.5% of Cs, while it was conversely decreased by 0.1-0.3% and 1%, 1.1%, 1.3% and 1.5% of CS in MM9 medium. It seems that hns protein plays a key role in up/down pap expression regulation of UPEC in response to environmental stresses. However, the reason for how L. rhamnosus and L. crispatus are able to up-regulate or down-regulate pap expression is not clearly explainable due to numerous potent environmental factors in pap expression.
The lactobacillus strains GR-1 and RC-14 have long been known to inhibit uropathogen adhesion [15, 16]. This is assumed as a mechanism by which UPEC colonization is limited. The latest findings show that lactobacilli can induce stress on UPEC outer membrane and thereby adversely affect fimbriae structure and adhesion besides up-regulating the outer membranes of the two proteins OmpA and OmpX that play a role in stress response [13]. Lactobacillus presence appears to cause UPEC to produce porins so as to maintain osmotic balance and stability in the membrane. Both OmpA and OmpX are highly immunogenic [25] and their up-regulation may induce antimicrobial immune responses in the host. However, this test was not performed in this study. Yet, there is evidence of up-regulation of host antimicrobial factors following L. rhamnosuss GR-1 vaginal administer in a separate study [17, 10].
Discussion
Urogenital lactobacilli can antagonize UPEC strains, not necessarily through direct lethality, but via acidic growth inhibition, stress induction in the outer membrane, and environmental modification of a less conducive condition to UPEC thriving. This represents a further rationale for selecting probiotic lactobacilli that can prevent urogenital infections in women and finding new probiotic effects for the ecological treatment of vaginitis.
References
- Dielubanza E.J. Urinary tract infections in women. Med. Clin. North Am 2011; 95(1):27–41.
- Foxman B, Barlow R, D’Arcy H, Gillespie B, Sobel JD. Urinary tract infection: self- reported Incidence and associated costs. Ann. Epidemiol 2000; 10(3): 509–515.
- Stapleton A.E, Fennell C.L, Coder D.M, Wobbe C.L, Roberts P.L, Stamm W.E. Precise and rapid assessment of Escherichia coli adherence to vaginal epithelial cells by flow cytometry. Cytometry 2002; 50: 31-37.
- Delzell J, Lefevre M. Urinary tract infections during pregnancy. Am. Fam. Physician GP 2000; 61(3) : 713–721.
- Johnson JR, Stell AL.Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J. Infect Dis 2000; 181(2): 261-272.
- Connell I, Agace W, Klemm P, Schembri M, Marild S, Svanborg C. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc. Natl. Acad. Sci 1996; 93(2):9827–9832.
- Gonzalez PAA, Sanchez HG, Ponce R.R.E. Frequency, risk factors and vaginal colonization due to Escherichia coli. Ginecol Obstet Clin 2004; 72:68-75.
- Guyer DM, Gunther NW, Mobley HL. Secreted proteins and other features specific to uropathogenic Escherichia coli. J Infect Dis 2001;183:32-35.
- Van den Bosch JF, Emody L, Ketyi I. Virulence of haemolytic strains of Escherichia coli in various animal models. FEMS Microbiol. Lett 1982; 13(1):427–430.
- Zorc JJ, DA Kiddoo, KN Shaw. Diagnosis and management of pediatric urinary tract infections .Clin Microbiol Rev 2005; 18:417–422.
- Rippere Lampe KE, O’Brien AD, Conran R, Lockman HA. Mutation of the gene encoding cytotoxic necrotizing factor type 1 (cnf1) attenuates the virulence of uropathogenic Escherichia coli. Infect. Immune 2001; 69 (1): 3954–3964.
- Lane MC, Lockatell V, Monterosso G, et al. Role of motility in the colonization of uropathogenic Escherichia coli in the urinary tract. Infect. Immun 2005; 73(2): 7644–7656.
- Bahrain Mougeot, FK Buckles EL, Lockatell CV, et al .Type 1 fimbriae and extracellular polysaccharides are preeminent uropathogenic Escherichia coli virulence determinants in the murine urinary tract. Mol Microbiol 2002; 45(2) : 1079–1093.
- Schilling JD, Mulvey MA, Vincent CD, Lorenz RG, Hultgren SJ. Bacterial invasion augments epithelial cytokine responses to Escherichia coli through a lipopolysaccharide dependent mechanism. J. Immunol 2001; 166(1): 1148–1155.
- Torres AG, Redford P, Welch RA, Payne SM. TonB dependent systems of uropathogenic Escherichia coli aerobatic and heme transport and TonB are required for virulence in the mouse. Infect. Immun 2001; 69(3): 6179–6185,.
- Hagan EC, Mobley HLT. Uropathogenic Escherichia coli Outer Membrane Antigens Expressed during Urinary Tract Infection. Infect.Immun 2007; 75(8):3941–3949.
- Atlung T, H Ingmer. H-NS: a modulator of environmentally regulated gene expression. Mol Microbial1997; 24(2): 7-17.
- Blomfield C, The regulation of Pap and type 1 fimbriation in Escherichia coli. Adv. Microb Physiol 2001;45(2): 41–49.
- Normark S.Genetics of digalactoside-binding adhesion Escherichia coli. Infect Immun 2001; 41(2):942–949.
- Aoki SK, R Pamma, AD Hernday, JE Bickham, BA Braaten, DA Low .Contact-dependent inhibition of growth in Escherichia coli. Science 2005; 309(2): 1245–1248.
- Vogt J, Schulz GE. The structure of the outer membrane protein OmpX from Escherichia coli reveals possible mechanisms of virulence. Structure1999; 7(1):1301-1309.
- Wang Y. The function of OmpA in Escherichia coli. Biochem Biophys Res Commun 2002; 292(2): 396-401.
- Velraeds MM, van de Belt-Gritter B, van der Mei HC, Reid G, Busscher HJ. Interference in initial adhesion of uropathogenic bacteria and yeasts to silicone rubber by a Lactobacillus acidophilus biosurfactant. J Med Microbiol 1998; 47: 1081-1085.
- Gupta K, Stapleton AE, Hooton TM, Roberts PL, Fennell CL, Stamm WE. Inverse association of H2O2-producing lactobacilli and vaginal Escherichia coli colonization in women with recurrent urinary tract infections. J Infect Dis1998; 178(3) : 446-450.
- Pabich WL, Fihn SD, Stamm WE, Scholes D, Boyko EJ, Gupta K. Prevalence and determinants of vaginal flora alterations in postmenopausal women. J Infect Dis 2003; 188(2): 1054-1058.
- Badie G, DM Heithoff, MJ Mahan. LcrV synthesis is altered by DNA adenine methylase overproduction in Yersinia pseudotuberculosis and is required to confer immunity in vaccinated hosts. Infect Immun 2004; 72:6707–6710.
- Alakomi HL, Skytta E, Saarela M, Mattila-Sandholm T, Latva-Kala K, Helander IM. Lactic acid permeabilizes gram-negative bacteria by disrupting the outer membrane. Appl Environ Microbiol2000; 66(1): 2001-2005.







