Alexey Sergeevich Prokopyev, Anna Olegovna Martynenko, Tatiana Nikolaevna Kataeva and Yuliya Mikhailovna Pastukhova
National Research Tomsk State University, 36 Lenina Avenue, Tomsk, 634050, Russia
DOI : https://dx.doi.org/10.13005/bpj/531
Abstract
This paper provides seed anatomy, morphology and germination capacity analysis of some species in the subfamily Sedoideae cultivated in Siberian Botanical Garden of Tomsk State University. The research helped us identify the morphological characteristics of seeds in 21 species and 2 subspecies. We measured the main morphometric parameters as well as described the shape, coloration and surface type of seeds. The internal structure of seeds was analyzed and their germination capacity was determined after three months of storage.
Keywords
Sedoideae; seeds; morphology; germination capacity
Download this article as:| Copy the following to cite this article: Prokopyev A. S, Martynenko A. O, Kataeva T. N, Pastukhova Y. M. Seed Morphology and Germination Capacity of some Species in the Sedoideae subfamily (Crassulaceae family). Biomed Pharmacol J 2014;7(2) |
| Copy the following to cite this URL: Prokopyev A. S, Martynenko A. O, Kataeva T. N, Pastukhova Y. M. Seed Morphology and Germination Capacity of some Species in the Sedoideae subfamily (Crassulaceae family). Biomed Pharmacol J 2014;7(2). Available from: http://biomedpharmajournal.org/?p=3220 |
Introduction
The family Crassulaceae DC is a vast group of plants comprising over 30 genera and 1,500 species [1]. About 600 of these species belong to the subfamily Sedoideae Berger. Species of the family are found in virtually all parts of the world but some deserts, continental Antarctic and islands of the Pacific Ocean [2]. The centers of their diversity are Mexico, South Africa, Macaronesia, the Mediterranean region and Eastern Asia [3]. The life forms of the Crassulaceae representatives include herbs, semi-shrubs and sub-shrubs with different adaptations to dry growing conditions. A number of species are valuable medicinal, nectariferous and ornamental plants and their introduction is of particular interest.
Introduction research is generally aimed at enriching the gene pool of cultivated plants with new valuable taxa. By creating collections of wild flora species, we can resolve some issues associated with researching and maintaining biodiversity as well as using beneficial plant species.
In Siberian Botanical Garden of Tomsk State University, we did an open-air trial of various representatives of the subfamily Sedoideae: 9 species of the genus Phedimus Raf., over 20 species of the genus Sedum L., 13 specues of the genus Hylotelephium H. Ohba, 3 species of the genus Orostachys Fisch., and 10 species of the genus Rhodiola. Many of these have successfully passed introduction tests and are recommended for practical use [4].
Seed coat characters are stable, conservative, and almost unaffected by the environment, which gives them high taxonomic value [5, 6]. Therefore, the analysis of Sedoideae seed morphological traits is quite valuable for species identification, and studying germination parameters helps evaluate the reproductive capabilities of the species.
This research was aimed at analyzing the seed anatomy, mophology and germination capacity of some representatives of the subfamily Sedoideae from the genera Sedum, Rhodiola, and Orostachys.
Objects and methods
The laboratory research took place in 2011-2013. The objects of this research were 21 species and 2 subspecies of the subfamily Sedoideae: Hylotelephium erythrostictum (Miq.) H. Ohba, Hylotelephium ewersii (Ledeb.) H. Ohba, Hylotelephium populifolium (Pallas) H. Ohba, Hylotelephium telephium subsp. fabaria (W.D.J. Koch) H. Ohba, H. telephium subsp. maximum (L.) H. Ohba, Hylotelephium triphyllum (Haw.) Holub, Orostachys spinosa (L.) Sweet, Phedimus aizoon (L.) ‘t Hart, Ph. florifer (Praeger) ‘t Hart, Ph. hybridus (L.) ‘t Hart, Ph. kamtschaticus (Fisch.) ‘t Hart, Ph. middendorffianus (Maxim.) ‘t Hart, Ph. obtusifolius (C.A. Mey.) ‘t Hart, Ph. spurius (M. Bieb.) ‘t Hart, Ph. stolonifer (J.F. Gmel.) ‘t Hart, Rhodiola algida Fisch. & C.A. Mey., Rh. rosea L., Sedum acre L., S. album L., S. hispanicum L., S. reflexum L., S. sexangulare L.
The papers by Artyushenko [7], Danilova [8], Gontcharova [3], Brouwer and Staehlin [9] were used as reference to describe the seed morphology, which was inspected under MSP-1 stereoscopic microscope (Lomo, Russia) at ×16 and ×32 magnification. Being more practical, this approach is valuable for express diagnostics of seeds in crop seed lots. Seed cross sections were prepared on the MZ-2 freezing microtome (Tochmedpribor, Ukraine). Seed anatomy was assessed under Axio Lab A1 light microscope (Carl Zeiss, Germany) at ×50 and ×100 magnification. The images were received, processed and analyzed by means of Axio Vision 4.8 software.
The seed weight was measured on DX-200 electronic scale (A&D, Japan) graduated in 0.001 g. 500 seeds were weighted in triplicate. The data obtained were extrapolated to 1,000 seeds.
The seed coloration was described by comparing it with the scale developed by Bondartsev [10] for biological objects.
After 3 months of storage, the seed germination capacity was studied under laboratory conditions. The seeds were sprouted in Petri dishes on wet filtered paper, 100 pieces at a time, in triplicate, in TSO-1/80 thermostat (Smolenskoye SKTB SPU, Russia) at 24˚С. The experiment was held both in the light and in the dark.
Results and discussion
Among the representatives of the family Crassulaceae, seeds are generally numerous, small, ovate or oblong, and often spindle-shaped. The seed surface can be shiny or dull, reticulate, verrucate, longitudinally striate or corrugate [8].
Studies of the Sedoideae seed morphology have shown that the seeds in the species under study are small, 0.65 to l.78 mm long and 0.29 to 0.48 mm wide. The smallest and lightest seeds were found in Orostachys spinosa (0.025 g/1000 pcs) and the biggest and heaviest ones, in Rhodiola algida (0.133 g/1000 pcs).
Most of the species studied have obovate-shaped seeds. Also, Sedoideae seeds are typically oblong, oblong-obovate, oblanceolate and oblong-ovate in shape. The Phedimus middendorffianus seeds are also sickle-shaped. There is often a raphe in the shape of a narrow thin ridge located along the seed from the micropyle to chalaza. The seed coloration of the species under study is ocher yellow, bister, brown, dark brown, snuffy brown (Table 1, Figure 1). According to our observations as well as Gontcharova’s data [3], there are rugose or wing-like spermoderm projections at the chalazal end in the species of the genus Rhodiola and Hylotelephium. These are most likely adaptations for sind dissemination.
Sedoideae seed coat sculpture differs depending on the genus and even species. Gontcharova [3, 11] identifies 4 main seed surface types as illustrated by Sedoideae of the Russian Far East (longitudinally costate, reticulate, multipapillate, and colliculate). Orlova [12] singles out the following seed surface types for the representatives of Sedoideae introduced in the south-west of the Black Earth Region: colliculate, alveolate, corrugate, and corrugate-alveolate.
When describing the seed surface types, we were guided by the earlier developed approaches used for characterizing seed morphology and made some contributions to these approaches.
Thus, we have subdivided the Sedoideae species under study into the following groups by the seed surface type:
Longitudinally corrugate surface (corrugations are pronounced, reticulate pattern is hardly, if at all, noticeable): Hylotelephium ewersii, H. populifolium, Phedimus aizoon, Ph. florifer, Ph. hybridus, Ph. kamtschaticus, Ph. middendorffianus, Ph. obtusifolius, Ph. spurius, Ph. stolonifer, Rhodiola rosea, Sedum album, S. hispanicum, S. reflexum.
Longitudinally corrugate-reticulate surface (corrugations and reticulation between them are well-marked): Hylotelephium erythrostictum, Hylotelephium telephium subsp. fabaria, H. telephium subsp. maximum, Hylotelephium triphyllum.
Reticulate (or weakly reticulate) surface: Orostachys spinosa; Rhodiola algida.
Alveolate surface: Sedum acre.
Colliculate surface: Sedum sexangulare.
Table 1: Seed morphology in some representatives of the subfamily Sedoideae
| # | Species | Seed dimensions | Weight of 1,000 seeds, g | Coloration | Surface type | Shape | |
| length, mm | width, mm | ||||||
| 1 | Hylotelephium erythrostictum | 1.20±0.05 | 0.44±0.04 | 0.088±0.004 | bister | longitudinally corrugate-reticulate | oblong, oblanceolate |
| 2 | H. ewersii | 0.91±0.03 | 0.32±0.03 | 0.073±0.005 | bister | longitudinally corrugate | oblong |
| 3 | H. populifolium | 0.92±0.04 | 0.38±0.02 | 0.078±0.006 | bister, brown | longitudinally corrugate | oblong-obovate, oblanceolate |
| 4 | H.telephium subsp. fabaria | 1.06±0.05 | 0.32±0.02 | 0.063±0.002 | dark brown
bister |
longitudinally corrugate-reticulate | oblong, oblanceolate |
| 5 | H. telephium subsp. maximum | 1.36±0.07 | 0.50±0.01 | 0.093±0.005 | dark brown | longitudinally corrugate-reticulate | oblong, oblanceolate |
| 6 | H. triphyllum | 1.53±0.06 | 0.47±0.01 | 0.118±0.007 | bister, snuffy brown | longitudinally corrugate-reticulate | oblanceolate, oblong |
| 7 | Orostachys spinosa | 0.65±0.03 | 0.29±0.02 | 0.029±0.002 | ocher yellow, sand | weakly reticulate | ovate-oblong |
| 8 | Phedimus aizoon | 1.09±0.07 | 0.48±0.05 | 0.084±0.004 | dark brown | longitudinally corrugate | obovate |
| 9 | Ph. florifer | 0.81±0.01 | 0.31±0.03 | 0.087±0.005 | dark brown | longitudinally corrugate | obovate |
| 10 | Ph. hybridus | 0.82±0.02 | 0.37±0.05 | 0.069±0.003 | dark brown, bister | longitudinally corrugate | obovate |
| 11 | Ph. kamtschaticus | 0.94±0.04 | 0.40±0.02 | 0.079±0.006 | dark brown | longitudinally corrugate | oblong-obovate, obovate |
| 12 | Ph. middendorffianus | 0.88±0.03 | 0.42±0.06 | 0.079±0.004 | dark brown | longitudinally corrugate | obovate, sickle-shaped |
| 13 | Ph. obtusifolius | 0.69±0.05 | 0.41±0.01 | 0.086±0.007 | dark brown | longitudinally corrugate | obovate |
| 14 | Ph. spurius | 0.98±0.06 | 0.42±0.06 | 0.085±0.005 | dark brown | longitudinally corrugate | obovate, oblong-obovate |
| 15 | Ph. stolonifer | 0.68±0.04 | 0.31±0.01 | 0.045 ±0.003 | dark brown | longitudinally corrugate | obovate |
| 16 | Rhodiola algida | 2.62±0.36 | 0.60±0.04 | 0.133 ±0.009 | chestnut brown | reticulate | oblong, oblanceolate |
| 17 | Rhodiola rosea | 1.44±0.12 | 0.46±0.01 | 0.086±0.006 | dark brown | longitudinally corrugate | oblanceolate |
| 18 | Sedum acre | 0.79±0.04 | 0.35±0.03 | 0.059±0.002 | brown | alveolate | obovate |
| 19 | S. album | 1.01±0.09 | 0.33±0.02 | 0.064±0.003 | bister | longitudinally corrugate | oblong, oblong-obovate |
| 20 | S. hispanicum | 0.51±0.04 | 0.29±0.02 | 0.052±0.002 | dark brown | longitudinally corrugate | obovate |
| 21 | S. reflexum | 1.15±0.08 | 0.44±0.03 | 0.089±0.007 | dark brown | longitudinally corrugate | oblong-obovate, oblong |
| 22 | S. sexangulare | 0.60±0.04 | 0.29±0.03 | 0.030±0.002 | brown | colliculate | ovate, obovate |
 |
Figure 1: Seeds of some representatives of the subfamily Sedoideae
|
Sedoideae seed anatomy has uniform structure. The embryo is large and occupies almost the entire inner space of the seed; the endosperm is monolayer and almost absent or unstructured in most species at maturity. The spermoderm is composed of two double-layer coats [13, 14, 3]. The findings of morphological and anatomical research into the seeds of the model species in different genera of the subfamily Sedoideae also confirm literature data.
The research has established that the spermoderm pattern of most species studied has projections of different shape and length. The most prominent spermoderm pattern is observed in Phedimus aizoon (Figure 2), projections in Orostachys spinosa are not prominent. The seed coat thickness varies from 0.019 to 0.031 mm. Embryo size also varies over a wide range in the specimens studied, which directly correlates with the overall seed size. The largest embryo is observed in Rhodiola rosea (0.396 mm2) and the smallest one, in Orostachys spinosa (0.172 mm2) (Table 2).
Table 2: Embryo area in some Sedoideae representatives in longitudinal section
| # | Species | Embryo area, mm2 |
| 1 | Hylotelephium triphyllum | 0.264±0.044 |
| 2 | Orostachys spinosa | 0.172±0.021 |
| 3 | Phedimus aizoon | 0.276±0.033 |
| 4 | Ph. spurius | 0.207±0.025 |
| 5 | Rhodiola rosea | 0.396±0.051 |
| 6 | Sedum acre | 0.196±0.022 |
| 7 | S. reflexum | 0.269±0.032 |
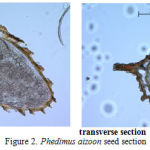 |
Figure 2: Phedimus aizoon seed section
|
The seeds of most species under study have high germinating property during the first year of storage [15]. After long-term storage, however, the germination capacity plummets, which, according to Gontcharova and Abankina [16], is due to the seed having undeveloped monolayer endosperm actively used by the large embryo.
We have established that, of all the Sedoideae studied, the following species have the highest germination performance after three months’ storage: Hylotelephium ewersii, H. populifolium, H. telephium subsp. maximum, H. triphyllum, Orostachys spinosa, Phedimus aizoon, Ph. florifer, Ph. hybridus, Ph. kamtschaticus, Ph. middendorffianus, Sedum acre (84-100%). Poor germination performance is observed in the seeds of Phedimus spurium, Rhodiola rosea and S. reflexum (23-40%) (Table 3). Kim [17] argues that poor germination performance of Rhodiola rosea seeds results from their thick seed coat inhibiting the access of oxygen and water to the embryo.
The seeds of most of the species under study are light-sensitive. The dark-germination percentage of many of the species studied is significantly lower. Seeds of some Sedoideae species tested do not germinate at all (Hylotelephium ewersii, H. telephium subsp. maximum, Sedum acre, S. reflexum). With Hylotelephium triphyllum, Phedimus aizoon, Ph. florifer and Ph. hybridus, light has no significant effect on seed germination (Table 3).
Table 3: Seed germination capacity of some Sedoideae
| # | Species | Germinated seeds | |
| in the light, % | in the dark, % | ||
| 1 | Hylotelephium erythrostictum | 60 | 16 |
| 2 | H. ewersii | 89 | 0 |
| 3 | H. populifolium | 100 | 68 |
| 4 | H.telephium subsp. fabaria | 68 | 32 |
| 5 | H. telephium subsp. maximum | 96 | 0 |
| 6 | H. triphyllum | 100 | 98 |
| 7 | Orostachys spinosa | 84 | 12 |
| 8 | Phedimus aizoon | 96 | 72 |
| 9 | Ph. florifer | 92 | 84 |
| 10 | Ph. hybridus | 84 | 68 |
| 11 | Ph. kamtschaticus | 100 | 12 |
| 12 | Ph. middendorffianus | 84 | 44 |
| 13 | Ph. obtusifolius | 68 | 4 |
| 14 | Ph. spurius | 40 | 12 |
| 15 | Rhodiola rosea | 23 | – |
| 16 | Sedum acre | 88 | 0 |
| 17 | S. album | 60 | 36 |
| 18 | S. hispanicum | 77 | 2 |
| 19 | S. reflexum | 34 | 0 |
Conclusion
We have established that the seeds of Sedoideae species are small or very small, 0.65-l.78 mm long and 0.29-0.48 mm wide. Seed shape varies from obovate to oblong. Seed coloration ranges from ocher yellow to dark brown. In terms of seed surface types, the Sedoideae species studied are subdivided into the following groups: longitudinally corrugate, longitudinally corrugate-reticulate, reticulate, alveolate, and colliculate. The largest embryo is observed in Rhodiola rosea and the smallest one, in Orostachys spinosa. The seeds of most of the species under study retain their high germination capacity after 3 months of storage; however, they are light-sensitive. Low germination rates were only observed in seeds of Phedimus spurius, Rhodiola rosea and Sedum reflexum.
Acknowledgments
This work was supported by the Ministry of Education and Science of the Russian Federation. State registration number of the theme: 114040740044.
References
- Borisova, A.G. (1939). Familia Crassulaceae DC. In: USSR flora. Bd.9. USSR Academy of Sciences, Moscow – Leningrad, pp. 9-134.
- Byalt, V.V. (2002) Familia Crassulaceae J. St.-Hil. In: East European flora. Bd.10. (SPCPA, Saint Petersburg, рр. 250-285.
- Gontcharova, S.B. (2006). Sedoideae (Crassulaceae) of the Russian Far East flora. Dalnauka, Vladivostok.
- Prokopyev, A.S. (2008). Biological features of species in the genus Sedum L. in the wild and when introduced in the forest area of Western Siberia, Ph.D. thesis, Tomsk State University, Tomsk.
- Barthlott, W. (1981). Epidermal and seed surface characters of plants: systematic applicability and some evolutionary aspects. Nord. J. Bot., 1: 345–354.
- Barthlott, W. (1984). Microstructural features of seed surfaces. In: Heywood, V.H., Moore, D.M. (Eds.), Current Concepts in Plant Taxonomy. Academic Press, London, pp. 95–105.
- Artyushenko, Z.T. (1990). Atlas of descriptive morphology in higher plants. Seed. Nauka, Leningrad.
- Danilova, M.F. (1996). Comparative anatomy of seeds, Bd.5. Mir i sem’ya, Saint Petersburg, рр. 25-32.
- Brouwer, W., Staehlin, A. (2010). Handbuch der Samenkunde fuer Landwirtschaft, Gartenbau und Forstwirtschaft mit einem Schluessel zur Bestimmung der wichtigsten landwirtschaftlichen Samen. KMK Publishing House, Moscow.
- Bontartsev, A.S. (1954). Color scale. USSR Academy of Sciences, Moscow – Leningrad.
- Gontcharova, S.B. Gontcharov, A.A., Yakubov, V.V., Kondo, K. (2009). Seed surface morphology in some representatives of the Genus Rhodiola sect. Rhodiola (Crassulaceae) in the Russian Far East. Flora – Morphology, Distribution, Functional Ecology of Plants, 204 (1): 17-24.
- Orlova, O.N. (2011). Biological features of species and sections of the subfamily Sedoideae Berger (Crassulaceae DC.) when introduced into the south-west of Black Earth Region, Ph.D. thesis, Belgorod State National Research University, Belgorod.
- Berger, A. (1930). Crassulaceae DC. In: Die natürlichen Pflanzenfamilien. Berlin. Aufl. 2, pp 352-483.
- Knapp, U. (1994). Morphology of the seed-coat and the system of the Crassulaceae family. Botanische Jahrbuecher fuer Systematik Pflanzengeschichte und Pflanzengeographie, 116(2): 157-187.
- Prokopyev, A.S., Belyaeva, T.N., Konusova, O.L. (2008). Reproductive biology of species in the genera Sedum and Hylotelephium (Crassulaceae) when introduced in the south of Tomsk Region. Plant Resources, 44 (1): 31-39.
- Gontcharova, S.B., Abankina, M.N. (1999). Features of seed structure and germination in some Far-Eastern species of the genus Sedum L. Plant Resources, 35(1): 46-52.
- Kim E.F. (1999). Rhodiola rosea (roseroot) and biological basis of its introduction in the crop. Altai State University, Barnaul.







