Padera Faryadyan1, Afra Khosravi2, Meysam Kashiri3 and Reza Valizadeh4
1Psychosocial Injuries Research Centre, Ilam University of Medical Sciences, Ilam, IIran.
2Department of Immunology, Faculty of Medicine, Ilam University of Medical Sciences.
3Department of Hematology, Allied Medical School, Tehran University of Medical Sciences, Tehran, Iran.
4Department of Psychology, Faculty of Medicine, Ilam University of Medical Sciences, Ilam, Iran.
DOI : https://dx.doi.org/10.13005/bpj/462
Abstract
There are many studies indicating that prenatal condition influences offspring different behaviors. In this study we investigated the effect of prenatal exposure to different colors on variations of serum testosterone levels in adult male offspring rats. Four groups of pregnant female Wistar rats, seven rats in each group, were enrolled in this study. The pregnant rats were placed in color chambers including green, blue, black and white (control). After delivery, male offspring of all color groups transferred to clear Plexiglas’s chambers until age 8-9 weeks and their serum testosterone concentration were measured using the ELISA Kit.The serum testosterone level was significantly high in adult male offspring’s who were prenatally exposed to blue and green color compared to male offspring’s who were prenatally exposed to black and white colors. Increasing of serum testosterone levels was also significant in adult male rats who were prenatally exposed to blue color. The results showed serious changes of testosterone in male offspring’s due to prenatal exposure to different colors which can be translated as testosterone variables. We can document that stimulation during pregnancy can lead to testosterone changes in male offspring and such changes can be made by colors.
Keywords
Testosterone; Prenatal; Male offspring; Color; Rat; ElISA Kit
Download this article as:| Copy the following to cite this article: Faryadyan P, Khosravi A, Kashiri M, Valizadeh R. Prenatal Exposure to Blue and Green Colors Increases Serum Testosterone Levels in Male Offspring Rats. Biomed Pharmacol J 2014;7(1) |
| Copy the following to cite this URL: Faryadyan P, Khosravi A, Kashiri M, Valizadeh R. Prenatal Exposure to Blue and Green Colors Increases Serum Testosterone Levels in Male Offspring Rats. Biomed Pharmacol J 2014;7(1). Available from: http://biomedpharmajournal.org/?p=2872 |
Introduction
Testosterone is a steroid hormone synthesized by Leydig cells in ovaries in females and the testes in males and adrenal glands in both sexes. Testosterone biosynthesis contemporarily occurs with the spermatogenesis and fetal Leydig cell differentiation in male rats. Vivo models were performed to study the hormonal adjusting of spermatogenesis by testosterone including hypophysectomy, hormone suppression, and hormone-restoration (Huang et al., 1987; Sun et al., 1989; O’Donnell et al., 1994). Animal study showed that testosterone secretion during late pregnancy and neonatal periods provokes magnified brain sexual dimorphism in rats. This finding was discovered in both endocrinology and sex-specific behaviour in adults (Sakuma, 2009). Experimental result of birds showed effective variables on changes in testosterone levels, such as type of territoriality, breeding season length, and altitude; the higher the testosterone levels, the shorter the breeding season and the increase of testosterone levels in high altitude (Goymann et al., 2004). The research demonstrated effective environmental factors, such as day length on changes of testosterone levels in birds in a photoperiod of 12 h of light and 12 h of darkness (12 Light, 12 Dark), female bird testosterone levels reduced after the last egg of the clutch was laid while level of testosterone decreased after the first egg was laid in a photoperiod of 16 Light 8 Dark. Testosterone concentrations changed significantly in reaction to alter photoperiod. In the end, the level of testosterone in the yolk of individual eggs was positively equal with the concentrations of testosterone in the female during the yolk phase of each egg. The above-mentioned study suggests that there is a relationship between environmental conditions from mother to offspring, and improving mechanisms can adjust reproduction and offspring behavior (Hubert, 1996).
The experimental investigation has revealed that the temporal patterns of testosterone levels in blood can differ among populations and individuals. Testosterone adjusts and modifies reproductive behavior (both sexual and aggressive) and mating systems. So the pattern and amplitude of change in testosterone levels are significantly beneficial in provoking predictions about hormonal characteristics of mating systems and breeding conditions (Wingfield, Hegner, Dufty & Ball, 1990). A study assessing important genetic and environmental effects on behavior indicated that there were no significant difference between genetic and environmental effects on males and females (Rhee et al., 2002). The evidence show that effective environmental factors which are determinant and significant on behavior, can also alter it (Paul & Stern, 2000). The researchers who studies on rhesus monkeys revealed effectiveness of several social and environmental variables on circulating testosterone concentrations. Independent and interaction effects were displayed. The factors were investigated to influence concentrations of circulating testosterone in this study included successful and unsuccessful agonistic encounters, ontogenetic status, relative access to females circadian rhythms, season (seasonal mating typical of rhesus) and alterations in social rank (Irwin et al., 1974). The result has revealed that moderate prenatal psychological stress has significant effects on HPA axis function, testosterone level, growth and behavior in male offspring’s (Amita Kapoor et al., 2005). It is obvious that maternal and prenatal stress can affect childhood behavior (O’Connor). The result of previous research suggests that prenatal receiving of betamethasone probably reduces testosterone peak in male pups by enhancing maternal corticosteroid concentration. This peak is vital to brain for sexual differentiation and decreasing testosterone level in adulthood and leads to altered partner preference and sexual behavior (Renata et al., 2009). No studies have examined the effect of prenatal exposure to different colors on changing testosterone level in adult male offspring in human or animal models. This study investigated effects of prenatal exposure to different colors as environmental factors on changes of testosterone level in adult male offspring’s of rats.
Animals
The experiment was conducted on four groups (seven in each) of Wistar pregnant female rats weighed 200-250g. The rats were kept in a 25±2 °C temperature with a 12 hr. light /dark cycle and fed with standard diet and tap drinking water. Rats were purchased from the animal house of Ilam University. All procedures were approved by the division of Animal house, and also the ethical committee of Ilam University of Medal Sciences.
Procedure of rats’ prenatal exposure to different colors
Beginning on day 1of gestation, four groups (seven animals each) of Wistar pregnant female rats were transferred to color chambers constructed of clear Plexiglas’s covered by different colors including green, blue, black and white (control) (16 inches × 20 inches with 8-inch-high walls). The surrounding walls, floor and roofs of each chamber were all painted the same color as the chamber color according to the classification of working groups. After delivery, male offspring of all color groups were transferred to clear Plexiglas chambers until age 8-9 weeks, when their serum testosterone was extracted. Evidence showed that plasma testosterone levels increased dramatically between 5 weeks and 9 weeks of age as the rat became mature (Resko et al., 1968).
Color wavelengths
The blue and green colors were analyzed with thin-layer chromatography (TLC) for quantitative wavelengths determinations. The wavelengths of blue, green colors were 450 and 490 nanometers respectively as indicator of light color and black color was used as indicator of dark color while white color was used for the control group.
Blood collection, hormone assay
The serum samples in age 8-9 weeks of all adult male offspring’s were collected for the measurement of testosterone. Testosterone was assayed in each rat serum sample using the DRG international GMBH ELISA kit (Germany). The procedure of testosterone measurement is summarized below
Testosterone ELISA
Using disposable tips, 10 microliter of each calibrator and sample was transferred to appropriate wells. Then 100 microliter of incubation buffer was added into each well. Next, 50 microliter of enzyme conjugate was dispended into each well and the wells were incubated for 60 minutes at room temperature on a microplate mixer. Then the microplate was shaked. After that, the content of the wells were removed and they were rinsed 4 times with diluted washsolution (300 microliter per well). Wash solution was discarded as much as possible by beating the microplate on absorbent paper, and then 200 microliter of substrate solution was added to each well and was incubated for 30 minutes without shaking in the dark. The reaction ended by adding 50 microliter of stop solution to each well. The absorbance of each well at 450 nm was determined and the wells were read within15 minutes.
Statistical analysis
ANOVA was used to compare testosterone in each group of 7 rats. Normality of data in each group was checked using one sample kolmogroror- simirnov test. Dennett test was performed as a post hoc multiple comparison analysis, P-values less than 0.05 were considered significant.
Results
The effect of prenatal exposure to different colors on serum testosterone level in male adult offspring’s
Figure 1 displays the effects of prenatal exposure to different colors on serum testosterone level in male adult offspring’s. ANOVA indicated that the differences between various groups were significant (p < 0.05). Dunnett- test analysis (Table 1) showed that serum testosterone level of adult males who were prenatally exposed to black color was not significantly different from that of adult males who were exposed to white color. On the other hand, the results showed that the mean level of serum testosterone of adult male offspring’s who were prenatally exposed to black color was significantly different from adult males who were prenatally exposed to blue and green colors (p < 0.05). The difference between serum testosterone level of adult males who were prenatally exposed to white color and adult males who were prenatally exposed to blue and green colors was statically significant (p < 0.05). The results of our study demonstrated that the difference between adult male who were prenatally exposed to blue color and adult males who were prenatally exposed to green color was statically significant (p < 0.05). It can be seen that increasing amount of serum testosterone level was significant in adult males who were prenatally exposed to blue and green colors as compared to the controls (white) (Table1).
Table 1: The effect exposure prenatal to different colors on changes level serum testosterone in male adult off spring
| Dependent Variable | groups | Mean | Std. Deviation | Std. Error | 95% |
| confidence | |||||
| Serum testosterone | White(control) | 2.19 | 1.426 | 0.539 | |
| 3.5 | |||||
| Black | 2.01 | 0.967 | 0.365 | ||
| 2.91 | |||||
| Blue | 17.03 | 1.742 | 0.659 | ||
| 18.64 | |||||
| Green | 9.9 | 4.652 | 1.758 | 14.2 |
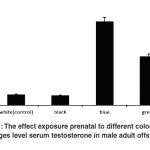 |
Figure 1: The effect exposure prenatal to different colors on changes level serum testosterone in male adult off spring
|
Discussion
The findings of our study showed that serum testosterone level was high in male adult offspring’s who were prenatally exposed to blue and green colors, while serum testosterone level was low in male adult offspring’s who were exposed to white and black colors as compared to blue and green colors. The data showed that the level of serum testosterone significantly increased in male adult offspring’s who were exposed to blue color during prenatal. It means that blue color provoked significant changes in the level of serum testosterone in adult male. It can be concluded that color as an environmental factor can effect on pregnancy conditions and cause changes of male offspring hormones such as testosterone. However, implicating mechanisms are unknown which requires future researches. Review of previous studies showed that mothers ‘condition during pregnancy effects on testosterone level and offspring’ behavior, and the male offspring’s who were exposed to stress during the trimester of pregnancy showed reductions in sexual behavior and fertility, while testosterone levels of the males exposed to stress did not differ from those of controls Crump and Chevins, 1989). Measuring level of testosterone in plasma male rat fetuses of mothers who had exposure to stress and control mothers on days 17, 18, 19, 21, and 23 (the day of birth) after pregnancy revealed that testosterone level in fetuses of stressed mothers were highest on day 17, felled on days 18 and 19, and then was stable, the absence of a surge of circulating testosterone during days 18 and 19 after gestation could have impaired masculine sexual behavior in male offspring’s of mothers who were exposed to stress, a period vital in the development of the rat CNS (Ingeborg, 1980). Investigating the effect of prenatal exposure to nicotine showed increase of plasma testosterone levels in adolescent female rat offspring’s and acutely in both male and female ovine fetuses of pregnant rats who received nicotine from day 4 until the end of gestation (Smithet al., 2003). Besides, studies show that mothers ’ condition effects on amount of saving testosterone in the eggs and the development of the chicken. A study revealed that when female birds mated to more attractive males, stored higher levels of testosterone and 5α-dihydrotestosterone in their eggs (Diego et al., 1999). The result shows that moderate prenatal psychological stress has significant effects on HPA axis function, testosterone level, growth and behaviour in male offspring. The study indicated importance of the timing of prenatal stress which is vital in determining the endocrine and behavioural phenotype in offspring’s. Stress during the period of maximal fetal brain growth displayed reducing plasma testosterone concentrations, surge of basal plasma cortisol levels and enhancing anxiety-related behaviour in offspring’s (Amita Kapoor et al., 2005). Testosterone has a preventing effect on HPA axis activity by reducing arginine vasopressin (AVP) in the median eminence (Viau HYPERLINK “http://onlinelibrary.wiley.com/doi/10.1113/jphysiol.2005.090191/full”&HYPERLINK “http://onlinelibrary.wiley.com/doi/10.1113/jphysiol.2005.090191/full” Meaney, 1996). Testosterone also has anxiolytic effects (Edinger & Frye, 2004) and inhibition testosterone metabolism in the hippocampus impedes these effects (Frye & Edinger, 2004). The studies on humans have demonstrated a significant incidence of behavioral disturbances in children who were prenatally exposed to psychological stress such as marital and familial problems, fear of impending war or death of husband (Stott, 1973; Meier, 1985; Huttenen & Niskanen, 1987). The finding of animal studies have displayed that stress during pregnancy provoke behavioral abnormalities such as impaired sexual function in offspring’s (Ward & Reed, 1985). In emphasis other studies found that testosterone levels of males reached a peak between November and January and fall from January to March with the decline in pituitary gonadotropin function ( Frederic and Roland ,1953). If visual signals are used in mate tendency in forests, it will affect reproductive behavior because forests indicate a mosaic of different spectral environments, so the appearance of a visual signal associates with joint effects of limitary light and the animal’s reactions spectra. A study investigated “function of ambient light spectra during displays and the reflectance spectra of color pattern elements” of three lekking birds and their visual backgrounds. The color patterns and behavior of each species maximized its visual contrast during its show and decreased it. Results showed important implications for the evolution of color patterns and display behavior. (Endler & Thery, 1996). Visual signals mediated through differential color patterns often activate signify the control of agonistic behaviour in fish (Huntingford & Turner, 1987). Background matching is well known in fish, and differential environmental background colours by manipulating body colour of fish demonestred in pairs of fish who were exposed to white background, both fish were initially pale in coloration and showed a high level of aggressive behaviour. However, the frequency of aggressive interactions declined over time. The pairs placed on a black background over time decrease of aggressive behavior was less obvious than those interacting on the white background (Abbott et al., 1985; O’Connor et al., 1999; Höglund et al., 2000).
Conclusion
According to above-mentioned studies prenatal condition can cause changes in testosterone level. Many studies have demonstrated the effects of prenatal stress on offspring’s development, behavior and alterations of hormones such as testosterone while there are few studies investigating variables that can have positive effects on peregenat mothers and their offspring’s condition. However, it is mentioned that stable and calmer condition of mothers during pregnancy have positive effect on offspring development, behavior and hormone changes, so more diverse studies are recommended to discover effective variables during prenatal period for inducing positive changes in offspring development and behavior. Few researches have investigated impact of color therapy on offspring development. To fill the gap of researches about color therapy, the present study was done to determine effect of prenatal exposure to different colors on changes in testosterone serum level. According to our study ,exposing mothers to green and blue color have positive effects on changes of testosterone concentration in serum of male adult offspring while prenatal exposure to white and black color had little impact on changing testosterone level. This result can open new gate to more studies of color therapy on development of offspring, and can conclude that color therapy during pregnancy such as blue and green color can be effective treatments for hormone disturbance such as testosterone. we hope to apply this method to human in future researches, to implicate mechanisms of provoking changes in testosterone levels by color therapy.
Reference
- Abbott, J. C., Dunbrack, R. L., & Orr, C. D. (1985). The interaction of size and experience in dominance relationships of juvenile steelhead trout (Salmo gairdneri). Behaviour/241-253.
- Bernstein, I. S., Rose, R. M., & Gordon, T. P. (1974). Behavioral and environmental events influencing primate testosterone levels. Journal of Human Evolution/3(6), 517-525.
- Crump, C. J., & Chevins, P. F. D. (1989). Prenatal stress reduces fertility of male offspring in mice, without affecting their adult testosterone levels.Hormones and behavior/ 23(3), 333-343.
- EdingerK.L&Frye,C.A.(2007). Androgens effects to enhance learning may be mediated in part through act at estrogen receptor-beta in the hippocampuse.Neurobiology of learning and memory/87(1),78-85.
- Endler, J. A., & Thery, M. (1996). Interacting effects of lek placement, display behavior, ambient light, and color patterns in three Neotropical forest-dwelling birds. American Naturalist/ 421-452.
- Gil, D., Graves, J., Hazon, N., & Wells, A. (1999). Male attractiveness and differential testosterone investment in zebra finch eggs. Science/ 286(5437), 126-128.
- Goymann, W., Moore, I. T., Scheuerlein, A., Hirschenhauser, K., Grafen, A., & Wingfield, J. C. (2004). Testosterone in tropical birds: effects of environmental and social factors. The American Naturalist/ 164(3), 327-334.
- Höglund, E., Balm, P. H., & Winberg, S. (2000). Skin darkening, a potential social signal in subordinate Arctic charr (Salvelinus alpinus): the regulatory role of brain monoamines and pro-opiomelanocortin-derived peptides. Journal of Experimental Biology/203(11), 1711-1721.
- Huang, H. F. S., Marshall, G. R., Rosenberg, R., & Nieschlag, E. (1987). Restoration of spermatogenesis by high levels of testosterone in hypophysectomized rats after long-term regression. Acta endocrinologica/ 116(4), 433-444.
- Huntingford, F. A., Turner, A. K., & Downie, L. M. (1987. Animal conflict. Chapman & Hall/CRC.
- Huttunen, M. O., & Niskanen, P. (1978). Prenatal loss of father and psychiatric disorders. Archives of general psychiatry/35(4), 429.
- Frederick ,G.,& Roland,K.(1953).Seasonal variation in testis stimulating.Ank/70(3),350.
- Frye, C. A., & Edinger, K. L. (2004). Testosterone’s metabolism in the hippocampus may mediate its anti-anxiety effects in male rats. Pharmacology Biochemistry and Behavior/78(3), 473-481.
- Kapoor, A., & Matthews, S. G. (2005). Short periods of prenatal stress affect growth, behaviour and hypothalamo–pituitary–adrenal axis activity in male guinea pig offspring. The Journal of physiology/566(3), 967-977.
- Meijer, A. (1985). Child psychiatric sequelae of maternal war stress. Acta Psychiatrica Scandinavica/72(6), 505-511.
- O’Connor, K. I., Metcalfe, N. B., & Taylor, A. C. (1999). Does darkening signal submission in territorial contests between juvenile Atlantic salmon, Salmo salar?. Animal Behaviour/ 58(6), 1269-1276.
- O’Connor, T. G., Heron, J., Golding, J., & Glover, V.( 2003). Maternal antenatal anxiety and behavioural/emotional problems in children: a test of a programming hypothesis. Journal of Child Psychology and Psychiatry.44(7)/1025-1036.
- O’Donnell, L., McLachlan, R. I., Wreford, N. G., & Robertson, D. M. (1994). Testosterone promotes the conversion of round spermatids between stages VII and VIII of the rat spermatogenic cycle. Endocrinology/135(6), 2608-2614.
- Piffer, R. C., Garcia, P. C., & Pereira, O. (2009). Adult partner preference and sexual behavior of male rats exposed prenatally to betamethasone. Physiology & behavior/98(1), 163-167.
- Prayitno, D. S., Phillips, C. J., & Omed, H. (1997). The effects of color of lighting on the behavior and production of meat chickens. Poultry science/76(3), 452-457.
- Resko, J. A., Feder, H. H., & Goy, R. W. (1968). Androgen concentrations in plasma and testis of developing rats. Journal of Endocrinology/40(4), 485-491..
- Rhee, S. H., & Waldman, I. D. (2002). Genetic and environmental influences on antisocial behavior: a meta-analysis of twin and adoption studies. Psychological bulletin/128(3), 490.
- Sakuma, Y.( 2009). Gonadal steroid action and brain sex differentiation in the rat. Journal of neuroendocrinology/21(4), 410-414.
- Schwab, H. (1996). Environment modifies the testosterone levels of a female bird and its eggs. Journal of Experimental Zoology/276(2), 157-163.
- Stern, P. C. (2000). New environmental theories: toward a coherent theory of environmentally significant behavior. Journal of social issues/56(3), 407-424.
- Stott, D. H. (1973). Follow‐up Study from Birth of the Effects of Prenatal Stresses. Developmental Medicine & Child Neurology/15(6), 770-787.
- Sun, Y. T., Irby, D. C., Robertson, D. M., & De Kretser, D. M. (1989). The effects of exogenously administered testosterone on spermatogenesis in intact and hypophysectomized rats. Endocrinology/125(2), 1000-1010.
- Smith, L. M., Cloak, C. C., Poland, R. E., Torday, J., & Ross, M. G. (2003) offspring. Nicotine & tobacco research/ 5(3), 369-374.
- Viau, V., & Meaney, M. J. (1996). The inhibitory effect of testosterone on hypothalamic-pituitary-adrenal responses to stress is mediated by the medial preoptic area. The Journal of neuroscience/16(5), 1866-1876.
- Ward, I. L., & Reed, J. (1985). Prenatal stress and prepuberal social rearing conditions interact to determine sexual behavior in male rats. Behavioral neuroscience./99(2), 301.
- Ward, I. L., & Weisz, J. (1980). Maternal stress alters plasma testosterone in fetal males. Science. 207(4428): 328-329. doi: 10.1126/science.7188648.
- Wingfield, J. C., & Grimm, A. S. (1977). Seasonal changes in plasma cortisol, testosterone and oestradiol-17β in the plaice, Pleuronectes platessa L. General and comparative endocrinology/31(1), 1-11.
- Wingfield, J. C., Hegner, R. E., Dufty Jr, A. M., & Ball, G. F. (1990). The” challenge hypothesis”: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. American Naturalist/ 829-846.







