Raina Jadhav1 and Sangeeta Parihar2
1Department of Chemistry, ISLE, IPS Academy, Indore, India.
2Department of Chemistry, Jai Narayan Vyas University, Jodhpur, India.
DOI : https://dx.doi.org/10.13005/bpj/489
Abstract
The fat obtained from the shade dried plant Malachra capitata (Linn) was saponified and the unsaponifiable matter was isolated. The unsaponifiable matter was identified as β - sitosterol by chemical and spectroscopic analysis.
Keywords
Malachra capitata (Linn); N.O.- Malvacaea; unsaponifiable; matter; β-sitosterol
Download this article as:| Copy the following to cite this article: Jadhav R, Parihar S. Isolation and Study of B-sitosterol the Unsaponifiable Matter from the Plant Malachra capitata (Linn). Biomed Pharmacol J 2014;7(1) |
| Copy the following to cite this URL: Jadhav R, Parihar S. Isolation and Study of B-sitosterol the Unsaponifiable Matter from the Plant Malachra capitata (Linn). Biomed Pharmacol J 2014;7(1). Available from: http://biomedpharmajournal.org/?p=2962 |
Introduction
The plant Malachra capitata Linn in commonly known as Ranbhendi in Bombay. It belongs to natural order Malvaceae.
The plant occurs in thought the hotter parts of India. It is used as emollient.
Experiment
About 500 gm the plant Malachra capitata (Linn) was shade dried, powdered and extracted with petroleum ether (40-600) in a Soxhlet extract for about 70hrs.
The petroleum ether (40-600) extract was concentrated to a get a yellow viscous fat.
About 50 gm of the above fat was saponified by refluxing it with alcoholic potassium hydroxide. The soap thus obtained was dissolved in water and the unsaponified matter was recovered by extraction with ether in a separating funnel.
Study of the Unsaponifiable Matter
The ether from this ethereal extract containing the unsaponifiable matter was distilled under reduced pressure to get the unsaponifiable matter which was when dried over anhydrous sodium sulphate, gave a light yellow product.
On CH, analysis, the unsaponifiable matter analysed for molecular formula C29H50O, M+ = 414 , m.p. 134-350C and (….)D22 = – 360 (in CHCl3).
The unsaponifiable matter also responded positively to following; colour reactions, which are characteristic of steroids.
A Yellow colour changing to red in Salkowski reaction2,
A violet red colour in Tschugajew reactions3,
Intense pink colour changing to violet in Noller’s reactions4 and
A red violet colour in Liebermann-Burchard reaction5.
The unsaponifiable matter showed the wave length of maximum absorbance a t
MeOH
λ max = 209mn.
Significant bands were observed in the IR spectrum of the unsaponifiable matter and the structural units inferred with the help of available literature 6-9 were at; 3358 (-OH), 2908 (-CH3 – CH2), 1640 (>C = CH stretching), 1452 (-CH3), 1370, 1135, 1070, 1018 (Triterpenoidal) 958,996 (Cyclohexane ring), 850 cm-1 (>C = CH deforming).
KBr
The bant at νmax at 1640 cm-1 in the IR spectrum showed the presence of unsaturation in the unsaponifiable matter which was further confirmed because it gave positive test with TNM.
Another band at 3358 cm-1 indicated the presence of -OH group(s) in the compound. The number of -OH group(s) was determined by the acetylation of the unsaponifiable matter which were estimated by acetylation with Ac2O/Pyridine to get an acetylated derivative, having molecular formula C31H52O2, M+= 456 and (m.p. 143-440C). The percentage of acety1 group (10.64%) was estimated by the procedure of Wiesenberger10 as described by Belcher and Godbert11, when it showed the presence of only one – OH group in it.
The formation of acety1 derivative was further supported by the appearance of the acety1 absorption band at 1720 cm-1 in the IR spectrum of acety1 derivative and disappearance of the hydroxy1 absorption in the IR spectrum of the acetylated unsaponifiable matter. The presence of only one acetoxy1 group (3H) was also confirmed by the signal at δ = 2.03 in the 1H -NMR12-13 spectrum of the unsaponifiable compound.
The signal for the proton at δ = 4.526 further established the secondary nature of the hydroxy1 group.
The unsaponifiable matter when oxidized with chromic acid yielded an oxidation product, m.p. 133-340C, molecular formula C29H48O, and M+ = 412, which was found to give positive Zimmermann test and confirmed the presence of 3-keto group. This observation concluded that the- OH group must be at C-3 and further confirmed that it must be secondary14.
Result and Discussion
The deep sweep in the available literature 15-16 concluded that the unsaponifiable matter under examination was identical with the known compound β-sitosterol (mmp, Co-PC, Co-TLC) (I). The identity of the compound was further confirmed by mmp. Co-TLC with authentic sample of β-sitosterol. Compilation of all above facts finally identified the unsaponifiable matter as: β-sistosterol.
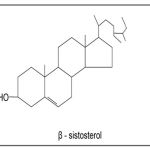 |
Figure a
|
Reference
- Chopra, R.N. Nayar, S.L. and Chopra, I.C. Glossary of Indian Medicinal plants C.S.I.R. publication page 160, (1956).
- Salkowski, E; Hoppe Sevlers, z. Vol. 57, page 52, (1908).
- Tschugajew, Chem Zig, Vol. 24, Page 542, (1900).
- Noller. C.R. : J. Amer. Chem. Soc. Vol. 64, page 3047, (1942).
- Liebermann, C.; Ber. Deash, Chem. Gas page 1804, (1885).
- Schwarz J. C.P. Physical Method in Organic Chemistry, Oliver and Boys, London p 80-113 (1964).
- Bellamy, L.J. ‘The Infra Red Spectra of Complex Molecules, Methuen and Co., Ltd., London (1945).
- Rao C.N.R. Chemical Applications of Infra Red Spectroscopy Acad, Press, London.
- Cross, A.A. “An introduction to Practical infrared Spectroscopy, Butter worth (1959).
- Weisenberger, Microchemie, 33 page, 51, (1947).
- Belcher, R. and Godbert, A.L.; Semi Micro quantitative organic analysis, page 164. (1954)
- Kalsi, P.S., Spectroscopy of Organic Compound, New Age International PVT (Ltd) Reprint (2003).
- Jackman, L.M., “Application of Nuclear Magnetic Resonance Spectroscopy in Organic Chemistry, Pergaman Press London.
- Barton, D.H.R. and Mayo, P.D.; J. chem.. Soc. Page 887 (1954)/
- Heilbron, I and Bunbury, H.M., ‘Dictionary of Organic Compounds’ Vol. IV. Page 361, (1953).
- Mishra, G.; Bhatnagar, S.C.; and Nigam, S.K.; Planta Medica, Vol. 31, page. 232-34, (1977).








