Om Prakash1 and S. A. Iqbal2
1Department of Chemistry, Saifia Science College, Barkatullah University, Bhopal - 462001, India.
2Crescent College of Technology Nabi Bagh, Karond, Bhopal - 462038, India.
DOI : https://dx.doi.org/10.13005/bpj/454
Abstract
The present study deals with the synthesis and hypoglycemic activity of Fe(II) and Zn(II) complexes with Pioglitazone hydrochloride a new oral antidiabetic drug. The conductometric titration using monovariation method indicate that complexes are ionic and L2M (ligand-metal ratio) type which is further confirmed by Job’s method of continuous variation as modified by Turner and Anderson. Analytical data and elemental analyses agree with the molecular formulae of complexes viz. [(C19H19N2O3S)+2 Fe(OH2)2]SO42- and [(C19H19N2O3S)2 Zn]2Cl- and geometry of complexes are assigned as octahedral and tetrahedral.The ligand and metal complexes have been tested on wistar albino rats in order to assess their hypoglycemic activity and found that metal complexes are more potent than parent drug.
Keywords
Pioglitazone; synthesis; complexes; hypoglycemic activity; alloxan method
Download this article as:| Copy the following to cite this article: Prakash O, Iqbal S. A. Hypoglycemic Study of Fe(II) and Zn(II) Complexes of Pioglitazone Hydrochloride on Wistar Albino Rats using Alloxan Induced Method. Biomed Pharmacol J 2014;7(1) |
| Copy the following to cite this URL: Prakash O, Iqbal S. A. Hypoglycemic Study of Fe(II) and Zn(II) Complexes of Pioglitazone Hydrochloride on Wistar Albino Rats using Alloxan Induced Method. Biomed Pharmacol J 2014;7(1). Available from: http://biomedpharmajournal.org/?p=2848 |
Introduction
Pioglitazone hydrochloride (PLZ) Fig.1 ((±) ‐5‐{p‐[2‐(5‐ethyl‐2‐pyridyl)ethoxy] benzyl}‐2, 4 ‐thiazolidinedione hydrochloride) is an oral antidiabetic agent used in the treatment of type 2 diabetes mellitus also known as non‐insulin‐dependent diabetes mellitus1 (NIDDM) or adult‐onset diabetes. PLZ decrease insulin resistance in the periphery and liver, resulting in increased insulin dependent glucose disposal and decreased hepatic glucose output. Currently, it is marked under the trade name Actos®2. It is a white or almost white crystalline, odourless powder, practically tasteless, insoluble in water and alcohols, but soluble in 0.1 N NaOH; it is freely soluble in dimethylformamide. It exhibits slow gastrointestinal absorption rate and inter individual variation of its bioavailability3. A survey of literature reveals that metal complexes of many drugs have been found to be more effective than the drug alone4 therefore, much attention is given to the use of thiazolidinedione hydrochloride due to their complexing nature with essential metals. Iron is vital for almost all living organisms by participating in a wide variety of metabolic process, including oxygen transport, DNA synthesis and electron transport. Some of the iron salts have long been in use in medicine, for example ferrous sulphide, ferrous sulphate, iron citrate etc. Iron therapy was mostly associated with the improvement of conditions of the blood. Since after the discovery of haemoglobin, an iron containing complex and different types of iron protien complexes like iron globunate etc. have been prepared. Chelates of iron, for example mono- and di-histidine ferro-metaphosphates, mono- and di-ornithine ferro-metaphosphates are described5. Recently, Iqbal and co-workers6-7 studied the iron complexes with sulphonylureas.
Zinc is an essential metal, so we get it through the food we eat. Zinc is most common mineral in the body and found in every plant and animal cell.8 It has been used since ancient times to heal wounds and plays an important role in the immune system, reproduction, growth, vision, blood clotting, proper insulin and thyroid function.9-10 Zinc complex of sulpha drugs have been prepared and studied by Salil et al., 11 Iqbal and co-workers 12-13 and found that the antidiabetic properties of zinc complex is more than the parent drug.
 |
Figure 1: Structure of Pioglitazone HCl
|
Experimental
Ligand- Metal ratio
To find out the ligand metal ratio, initially conductometric titration using monovariation method were carried out at 27±1 ºC and 0.005 M solution of PLZ was prepared in DMF (dimethylformamide). Similarly, solution of ferrous sulphate (FeSO4) and zinc chloride (ZnCl2)) were prepared in the ethanol of 0.01M concentration. A few drops of phosphoric acid were added to metal solution for their solubility. 20ml of ligand was diluted to 200ml with the same solvent. The ligand was titrated conductometrically against metal salt solution taken in burette using fraction of 1ml. Conductance was recorded after each addition with proper stirring. Results were plotted in the form of graph between corrected conductance and volume of metal salt added. From the equivalence point in the graph, ratio between ligand and metal were noted to be 2:1 (L2M).
Formation of these complexes in 2:1 (L2M) ratio were also confirmed by Job’s method14of continuous variation as modified by Turner and Anderson,15 Fig. 2 (a)-(b) and Fig.3 (a)-(b) conductance as index property, from these values the stability constant (log K) and free energy change (DF), were also calculated by using formula16-19 as;
 |
Figure 2 |
 |
Figure 3 |
Material and Reagents
All chemicals were used of analytical grade and of highest purity. They include pure sample of pioglitazone hydrochloride with molecular formula (C19H20N2O3S.HCl), received from Morepen Laboratories Limited Parwanoo, Distt. Solan (H. P.) India. The metal salt of FeSO4 and ZnCl2 obtained from Hi-media Laboratory, Mumbai, India.
Synthesis of Complexes
A weighed quantity of “PLZ” (2 mol) was dissolved separately in minimum quantity of DMF. Metal salt solution was prepared by dissolving separately in ethanol. Ligand solution was added slowly with stirring into the solution of metallic salt at room temperature, maintain the pH between 6.0 to 6.5 by adding dilute NaOH solution, on refluxing the mixture for 3-4 h at 70oC and on cooling, brown and white colored crystals were obtained which were filtered off, washed well with DMF and finally dried in vacuum and weighed.
Results and Discussion
The formation of metal complexes with organic compounds have long been recognized. The synthesized complexes are brown and white, being soluble in DMSO and insoluble in water, ethanol etc. Analytical data and conductometric studies suggest 2:1 (L2M) ratio. Physical and anylytical data of of PLZ metal complexes are given in Table 1. Structures of complexes are presented in scheme I (a)-(b).
 |
Scheme I: (a) PLZ- Fe complex (b) PLZ-Zn complex |
Hypoglycemic Study
Pharmacology is mainly concerned with the responses of living organisms to chemical stimuli. One may further divide the subject from a medical view point, into pharmacodynamics and pharmacotherapy, the former is concerned with the response of living organism to chemical stimuli in the absence of disease, while the later with the response of organism to such stimuli in a pathogenic state. This is the phase of pharmacology (i.e. pharmacotherapy) which is of special interest to the physician. Pharmacotherapy includes the treatment of the sick with drugs and therefore is of prime importance in practice of medicine. It is fundamental to the health-economy of the people. A compound or a complex which is to be recommended as a drug of utility must be capable of easy absorption and excretion. It is also essential that neither the absence itself nor the metabolic products thereof should exercise toxicity or any adverse side effect to the patient.
Animal Study
Where necessary such tests should be carried out on animals as rats, rabbits and dogs. When a substance has given satisfactory results for the aforesaid animals then only it may be tried on monkeys and men.
In present study we analyze the hypoglycemic activity on wistar albino rats
Animal care and handling
The anti-diabetic activity was carried out on wistar albino rats of 4 months, of both sexes, weighing between 130 to 180 gm. They were provided from Sapience Bio-analytical Research Lab, Bhopal, (M. P.). The animals were acclimatized to the standard laboratory conditions in cross ventilated animal house at temperature 25±2°C relative humidity 44 –56% and light and dark cycles of 12:12 hours, fed with standard pallet diet and water ad libitum during experiment. The experiment was approved by the institutional ethics committee and as per CPCSEA guidelines (approval no. 1413/PO/a/11/CPCSEA).
Chemicals
Alloxan monohydrate was purchased from Central Drug House (P) LTD. All other chemicals used for this study were of analytical grade.
Induction of diabetes by alloxan20-23
The diabetes was induced by a single intraperitoenal injection of a freshly prepared solution of Alloxan monohydrate (120 mg/kg b.w.). Blood samples were collected 72 hrs after Alloxan-injection. Rats with moderate diabetes having hyperglycemia (with blood glucose above 300 mg/dl) were taken for the experiment.
Experimental Design
In the experiment, a total of 33 rats were used. The rats were divided into 3 groups
comprising of 11 animals in each group as follows;
Group I
Rats served as diabetic control.
Group II
Rats received Pioglitazone- iron (PLZ- Fe) complex (10mg/kg, p.o.).
Group III
Rats received Pioglitazone-zinc (PLZ-Zn) complex (10mg/kg, p.o.).
Sample collection
Blood samples were collected through tail vein and blood glucose levels were estimated using an electronic glucometer (Gluco chek) and results are given in table 1.
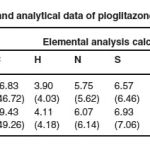 |
Table 1: Physical and analytical data of pioglitazone metal complexes.
|
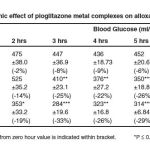 |
Table 2: Hypoglycemic effect of pioglitazone metal complexes on alloxan induced diabetic rats.
|
Statistical analysis
All the values are expressed as mean±standard error of mean (S.E.M.) and analyzed for ANOVA and posthoc Dun net’s t-test by employing statistical software, Graph Pad In Stat 3. Differences between groups were considered significant at *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Conclusions
For synthesis of complexes, the conductometric titration using monovariation method indicate that complexes is ionic and L2M (ligand-metal ratio) type, moreover stability constant and free energy change were also calculated by Job’s method of continuous variation as modified by Turner and Anderson. Analytical data and elemental analyses agrees to the molecular formulae viz. [(C19H19N2O3S)+2Fe(OH2)2]SO42- and [(C19H19N2O3S)2Zn]2Cl–. For evaluating the hypoglycemic activity of iron and zinc complexes of pioglitazone, three groups of wistar albino rats each having 11 animals of ± 150-200gm b.w. were selected which were given alloxan for making them diabetic. When all the three sets of animals A, B and C have attend a fixed blood glucose level ± 10mg/lit. the animals of the first group (A) were given distilled water only and the change in blood glucose level was recorded from zero hrs to eight hrs. Similarly group (B) animals were given pioglitazone-iron complex 10mg/kg b. w. and fall in blood glucose was recorded from zero to eight hrs. Similarly the group (C) animals were given zinc complex of pioglitazone and the value of fall in blood glucose were recorded from zero to eight hrs, all the results are summarized in table -1 which indicate that iron and zinc complexes of pioglitazone is capable of reducing the blood glucose level more. The efficacy of iron and zinc complexes is more as compared to drug may be explained on the basis that on complexation some of the bonds are broken while new bonds are formed like M-N, M-O etc. Moreover on the complexation, the size of the molecule reduces sufficiently as compared to drug molecule which causes the easy and quick absorption of the complexed drug in gastrointestinal tract and activating the pancreas for more and more release of insulin for antidiabetic activity. Results of the present work are also in conformity with the hypoglycemic effect of copper- phenformin complex over parent drug phenformin as mentioned by Piccini et al.
Acknowledgement
The author is thankful to the UGC New Delhi for financial assistance as RGNF. Authors are also thankful to Sapience Bio-analytical Research Lab, Bhopal, (M. P.) India.
References
- Abbasid, F., Lima, N. K. and Raven, G. M., Metabolism, 58: 373‐378 (2009).
- Lofty Saber AMR., J. Anal. Environ. Chem., 9: 118‐121 (2008).
- Kouichi, Res. Clin. Pract., 68:250-257 (2005).
- Sharma, S., Iqbal, S. A. and Bhattacharya, M., J. Chem., 25 (4): 1101-1104 (2009).
- Duychaerts, G., Analyst, 84: 201 (1959).
- Prakash, O., Iqbal, S. A. and Jacob, G., J. Chem., 29 (3):1079-1084 (2013).
- Jose S. and Jacob, G., J. Chem., 28 (2): 565-572 (2013).
- Mills, C. F., Zinc in hman Biology (Springer Verlag), (1989).
- Phipps, D. A., Metals and Metabolism, Oxford university press, P-63: (1976).
- Harrap, H. J., Arch Oral Biol., 29: 87 (1984).
- Salil, A. , Hamdani, Al. and Shaker, S. A., Orient. J. Chem., 27 (3): 835-845 (2011).
- Iqbal, S. A., Jose S. and Jacob, G., J. Chem., 27 (2): 731-735 (2011).
- Iqbal, S. A. and Zaafarny, I., J. Chem., 28(1):613-618 (2012).
- Job, P., Annales de Chimie, 10: 113 (1928).
- Turner, S. and Anderson, R. C., Journal of the American Chemical Society, 71: 912–914 (1949).
- Irving, H. and Rossotti, H. S., Chem. Soc., 3397 (1954).
- Irving, H. and Rossotti, H. S., Chem. Soc., 1176 (1955).
- Tawkir, M. and Zaafarany, I., J. Chem., 28 (4): 1697-1710 (2012).
- Prakash, O., Krishan, B. and Jacob G., J. Chem., 29 (2): 823-828 (2013).
- Mukherjee, P. K., Quality control of herbal drugs, an approach to evaluation of botanicals, Business horizons Pharmaceutical publishers, New delhi, India (2008).
- Krishan, B. and Zaafarany, I., J. Chem., 29 (4):1571-1577 (2013).
- Jyoti, M., Vihas, T.V., Ravikumar A. and Sarita, G., Glucose lowering effect of aqueous extract of Enicostemma Littorale Blume in diabetes: a possible mechanism of action. Ethno. harm., 81:317-20 (2002).
- Piccinni, F., Murazzi, E., Uberti and Lucatelli, , Pharmaco (pavia) Ed.sci. 15: 521(1960).








