Dao TA Nguyen and Tu HK Nguyen
School of Biotechnology, Hochiminh International University, Hochiminh city, Vietnam.
DOI : https://dx.doi.org/10.13005/bpj/449
Abstract
The aim of the present study was to determine whether water - soluble polysaccharides (EPS) of Lactococcus lactis NCR112 isolated in Phyllanthus urinaria could express the inhibition of human cancer cells as well as the antioxidant activity. The water - soluble polysaccharide of L. lactis NCR112 was studied on the antioxidant activity by using DPPH radical scavenging assay and sulforhodamine B (SRB) assay for anticancer activity tests on two HeLa and Hep G2 tumor cell lines. The highest crude EPS amount was obtained at the stationary phase. The crude EPS (10 mg/ml) showed the antioxidant activities with the inhibition percentage of 66.10 ± 1.157, equaled to the activity of ascorbic acid at 0.01315 µg/ml. The crude EPS of L. lactis NCR112 also showed the higher cytotoxicity percentage on HeLa (86.86 ± 4.875) than Hep G2 (50.36 ± 6.237). These results indicated that the exopolysaccharide isolated from L. lactis NCR112 constituted the major fraction that inhibited the proliferation of cancer cells. This was the first report on antioxidant activityof plant – originated L. lactis NCR112.
Keywords
Antioxidant activity; anticancer activity; DPPH radical scavenging assay; L. lactis NCR112; sulforhodamine B (SRB)
Download this article as:| Copy the following to cite this article: Nguyen D. T, Nguyen T. H. Detection on Antioxidant and Cytotoxicity Activities of Exopolysaccharides Isolated in Plant-Originated Lactococcus lactis. Biomed Pharmacol J 2014;7(1) |
| Copy the following to cite this URL: Nguyen D. T, Nguyen T. H. Detection on Antioxidant and Cytotoxicity Activities of Exopolysaccharides Isolated in Plant-Originated Lactococcus lactis. Biomed Pharmacol J 2014;7(1). Available from: http://biomedpharmajournal.org/?p=2831 |
Introduction
Most of diseases are due to the “oxidative stress” resulting from an imbalance between formation and neutralization of pro-oxidants. Oxidant stress is initiated by free radicals, which produced aerobic metabolism in the body, can cause oxidative damage of biological macromolecules such as proteins, lipids, and DNA in healthy human cells (Yen and Chen, 1995; Gutteridge and Halliwell, 1993; Halliwell, 1995). These changes contribute to oxidative stress that is among the major causative factors in the induction of many chronic and degenerative diseases including atheorosclerosis, ischemic heart diseases and diabetes mellitus, cancer, immunosuppression, neurodegenerative disease, ageing (Squadrito and Pryor, 1998; Devasagayam et al., 2004; Büyükokuroğlu et al., 2001; Shahidi et al., 1992; Gülçin et al., 2002; Branen, 1975), coronary heart disease and Alzheimer’s disease (Ames, 1983; Gey, 1990; Smith et al.,1996; Diaz et al., 1997). All human cells protect themselves against free radical damage by enzymes such as superoxide dismutase and catalase, or compounds such as ascorbic acid, toccopherol and glutathione (Niki, 1994). However, these protective mechanisms occurred by various pathological processes, and antioxidant supplements are necessary to combat oxidative stress. Currently, the well-known synthetic antioxidants like butylated hydroxylanisole (BHA) and butylated hydroxytoluence (BHT), tertiary butulated hydroquinon and galic acid esters, are reported to cause or promote releasing carcinogens. Therefore, the interest in the natural compounds with strong antioxidant properties has steadily been increasing. Furthermore, the plant-originated lactic acid bacteria such as L. rhamnosus PN04 showed the strong biological activities (Nguyen et al., 2013; 2014). Meanwhile, L. lactis strains isolated in milk and food has several beneficial effects, such as antimicrobial activity (Khalid et al., 2011; Elliott et al., 1996; Roy et al., 1996; Rodrıguez et al., 2005), ability to modulate immune response (Fang et al., 2000; Chatel et al., 2011), anti-tumorigenic activity (Shalke, 2013; Mohammadi, 2013) and antioxidant activity (Virtanen et al., 2007). It has been shown that L. lactis possessed antioxidant activity that was able to decrease the risk of accumulation of reactive oxygen species during ingestion of food (Pan and Mei, 2010; Rochat et al., 2005).
As the above stated reasons, study on the antioxidant and anticancer activities of plant – originated Lactococcus lactis NCR112 was necessary because of this strain adapted the harsh condition than the animal originated L. lactis.
Materials and Methods
Culture Lactococcus lactis NCR112 at difference growth phase
Lactobacillus lactis NCR112 isolated from Phyllanthus urinaria was deposited in DDBJ under the accession number (AB828399). This strain was cultured in De Man-Rogosa- Sharpe (MRS) (Biokar Diagnostics, Beauvais, India) and incubated at 37oC under aerobic conditions (pH 6.5). The optical density (OD) measurement at wavelength of 600 nm was performed every two hours.
Preparation of polysaccharides
Lactococcus lactis NCR112 was cultured in De Man-Rogosa- Sharpe (MRS) (Biokar Diagnostics, Beauvais, India) and incubated at 37oC under aerobic conditions. Cultures were collected at different phase of incubation and centrifuged at 10000 rpm for 30 min to separate the cell from the broth. The culture supernatant was let overnight to precipitate with three times volume of absolute cold ethanol (EtOH) and then centrifuged again at 10000 rpm for 30 min. The obtained pellet was resuspended with distilled water and further precipitated by adding three times volume of cold EtOH. The overnight solution was centrifuged to collect the water-soluble exopolysaccharides (EPS). The crude EPS was dried at 60oC to a constant weight. EPS stocks were dissolved in distilled water and then filtered through the 0.22 μm pore-size filters (Millipore, Bedford, Mass.) before using.
Antioxidant activity using DPPH radical scavenging assay
In order to perform the 2,2-diphenylpicrylhydrazyl (DPPH assay), amounts of 4.3 mg of DPPH were dissolved in 3.3 ml methanol in a test tube (Padmavathy, 2014). Solution was protected from light by covering the test tubes with aluminum foil. 150 ml of above solution were added to 3ml methanol. This solution was measured at 517 nm on UV spectrophotometer. Methanol was used as blank. This reading was used as control reading. For the test and standard (ascorbic acid), the aliquots of different concentration ranging were prepared. After 50 ml of tested EPS (10 mg/ml) and standard ascorbic acid in the various concentration were diluted with methanol up to 3ml, 150 ml DPPH was added. All these samples were taken after 12 h and measured at 517 nm on UV-visible spectrometer (Shimadzu, UV-1601, Japan). The DPPH free radical scavenging activity was calculated using the following formula:

Where As and Ac are the absorbance of control and sample, respectively.
Antitumor activity of sulforhodamine B (SRB) assay
In order to perform antitumor activity test, the crude EPS sample was prepared according to the above prescribed procedure. The sulforhodamine B (SRB) assay was used in the study according to the method of Longo-Sorbello with a slight modification (Longo-Sorbello et al., 2005). Each cancer cell line was seeded in a 96-well plate (1.0 x 104 cells per well). After 24h of incubation, the test samples (115.3 x 106 cfu/ml of cell-free supernatant and 20 mg/ml of crude EPS) were added to the cancer cells, 5% CO2 for 48 h at 37oC. For this incubation time, no significant differences were observed in the pH of medium. Thereafter, 50 μl of 50% TCA (4oC) was added to each well containing 200 μl of medium to reach a final concentration of 10% TCA in each well and plate the 96-well plate for 1h at 4oC to allow cell fixation. After 1 h of incubation, the culture medium in each well was removed and the plate was gently washed with water (200 μl/well) five times and dried at room temperature for 12-24 h. 0.2% SRB (w/v) solution was added after the time for incubation to each well and leave at room temperature for 5-20 min. Then, washing the plate with 1% acetic acid was performed five times in order to remove unbound SRB. Drying the plate for 12-24 h before adding 200 μl Trizma-base 10 mM in oder to solublize bound SRB is necessary. The last step is plating the 96-well plate on a plate shaker for at least 10 min. Absorbance was measured at 492 nm and 620 nm using an enzyme-linked immunosorbent assay plate reader (Molecular Devices, Synnyvale, CA, USA). DMSO was used as a negative control. The percentage of viable cells was calculated as follows:
Statistical analyses
The SPSS 16.0 software (SPSS Inc., Chicago, IL, USA) was used to calculate the means and standard deviations in any experiments involving triplicate analyses of any samples. The statistical significance of any observed difference was evaluated by one-way analysis of variance (One way ANOVA), using the Bonferront Mutiple Comparisons Test.
Results and Discussion
Polysaccharide collection
There have been many publications that have developed the procedure for EPS isolation. Most of these methods commonly use solvent such as acetone (Vincent et al., 2001; Lemoine et al., 1997) or ethanol (Rodriguez et al., 2008) to precipitate EPS. In this study, using ethanol for EPS precipitation, the crude EPS samples were collected and determined at four different phases (table 1, figure 1). Although there was less significant difference between growth phases, the weight at stationary phase is obtained highest amount (0.0525 g/5ml). That might also due to the best growth correlating the EPS biosynthesis of Lactobacillus lactis NCR112.
Table 1: Weight of EPS at different phase of L. lactis NCR112
| Growth phase | Weight of crude EPS (g/5ml) |
| Early exponential phase | 0.0328 ± 0.0022a |
| Late exponential phase | 0.0456 ± 0.0003b |
| Stationary phase | 0.0479 ± 0.0022b |
| Death phase | 0.0471 ± 0.0038b |
Results are mean values of triplicate determinations ± SD
The sample letters in the same column are not significant different (p <0.05)
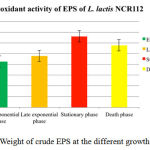 |
Figure 1: Weight of crude EPS at the different growth phases
|
Antioxidant activity tests using DPPH radical scavenging assay
In this present study, the antioxidant activity of the crude EPS of Lactobacillus lactis NCR112 at different phase was investigated using the DPPH scavenging assay. Scavenging activity of crude EPS produced at different phase on DPPH radical has been shown in Table 2 and Figure 2. The crude EPS (10 mg/ml) gave the antioxidant activities with the inhibition percentage of 52.86 ± 0.133 when compared with ascorbic acid activity at 13.15 μg/ml according to the standard curve (y = 1.7204x + 43.475, R2 = 0.9993). As a result, there was noticeable antioxidant activity of the EPS fraction.
Table 2: DPPH scavenging activity of crude EPS
| Growth phase | % DPPH scavenging activity of crude EPS (10 mg/ml) |
| Early exponential phase | 42.44 ± 0.576a |
| Late exponential phase | 47.71 ± 0.273b |
| Stationary phase | 66.10 ± 1.157c |
| Death phase | 57.62 ± 1.112d |
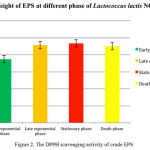 |
Figure 2: The DPPH scavenging activity of crude EPS
|
Antitumor activity on SRB assay
From the results of antioxidant activity, it was recognized clearly that the bacterium could produce the highest amount of antioxidant activity. Consequently, the crude EPS was performed to test antitumor activity. As illustrated in table 3 and figure 3, crude EPS was effective to Hela and Hep G2 cancer cells. Depending on the different effect mechanism to cancer cell lines, the cytotoxicity percentage of each sample was also different. For Hela cancer cell, the cytotoxicity percentage of crude EPS were 86.86 ± 4.875. In case of Hep G2 cancer cell, the cytotoxicity percentage of crude EPS were 50.36 ± 6.237. These results indicated that the exopolysaccharide isolated from L. lactis NCR112 constituted the major fraction that inhibits the proliferation of cancer cells. The cytotoxicity on the different cancer cell lines that may be affected by the cancer cell receptor binding. To clearly understand this point, more studies are being performed.
Table 3: Inhibitory effects of cell-free supernatant and crude EPS at stationary phase of L. rhamnosus PN04 on the growth of two human cancer cell lines
| Cancer cell line | % cytotoxicity of EPS |
| HeLa | 86.86 ± 4.875 |
| Hep G2 | 50.36 ± 6.237 |
Conclusion
The crude EPS derived from plant originated L. lactis NCR112 exerted significant antioxidant activity, as well as anticancer activity on two HeLa and Hep G2 cell lines. These polysaccharide components may be applied to various foods, and used as adjunction in cancer trials. In future, the optimizing conditions and components of EPS should be determined to exploit EPS for human health.
References
- Ames, B. N. (1983). Dietary carcinogens and anticarcinogens oxygen radicals and degenerative diseases. Science, 221(4617), 1256-1264.
- Branen, A. L. (1975). Toxicology and biochemistry of butylated hydroxyanisole and butylated hydroxytoluene. Journal of the American Oil Chemists’ Society, 52(2), 59-63.
- Büyükokuroğlu, M. E., GülçIn, I., Oktay, M., & Küfrevioğlu, O. I. (2001). In vitro antioxidant properties of dantrolene sodium. Pharmacological research: the official journal of the Italian Pharmacological Society, 44(6), 491-494.
- Chatel, J. M., Langella, P., Adel-Patient, K., Commissaire, J., Wal, J. M., & Corthier, G. (2001). Induction of mucosal immune response after intranasal or oral inoculation of mice with Lactococcus lactis producing bovine beta-lactoglobulin. Clinical and diagnostic laboratory immunology, 8(3), 545-551.
- Devasagayam, T., Tilak, J. C., Boloor, K. K., Sane, K., Ghaskadbi, S., & Lele, R. (2004). Free radicals and antioxidants in human health: current status and future prospects. The Journal of the Association Physicians India of physicals of India, 52, 794-804.
- Diaz, M. N., Frei, B., Vita, J. A., & Keaney Jr, J. F. (1997). Antioxidants and atherosclerotic heart disease. New England Journal of Medicine, 337(6), 408-416.
- Elliott, J. A., & Facklam, R. R. (1996). Antimicrobial susceptibilities of Lactococcus lactis and Lactococcus garvieae and a proposed method to discriminate between them. Journal of clinical microbiology, 34(5), 1296-1298.
- Fang, H., Elina, T., Heikki, A., & Seppo, S. (2000). Modulation of humoral immune response through probiotic intake. FEMS Immunology & Medical Microbiology, 29(1), 47-52.
- Gey, K.F. (1990). The antioxidant hypothesis of cardiovascular disease: epidemiology and mechanisms. Biochem. Soc. Trans.18, 1041-1045.
- Gülçin, I., Oktay, M., Küfrevioğlu, Ö. İ., & Aslan, A. (2002). Determination of antioxidant activity of lichen Cetraria islandica (L) Ach. Journal of Ethnopharmacology, 79(3), 325-329.
- Gutteridge, J. M., & Halliwell, B. (1993). Invited review free radicals in disease processes: a compilation of cause and consequence. Free Radical Research, 19(3), 141-158.
- Halliwell, B. (1995). How to characterize an antioxidant: an update. InBiochem Soc Symp, 61, 73-101.
- Khalid, K., Kiong, L. H., Chowdhury, Z. Z., & Khalid, K. (2011). Antimicrobial interaction of Lactococcus lactis subsp. lactis against some pathogenic bacteria. International Journal of Biosciences (IJB), 1(3), 39-44.
- Lemoine, J., Chirat,F., Wieruszeksi, J.M., Strecker, G., Favre, N., and Neeser, J.N. (1997). Structural characterisation of the exocellular polysaccharides produced by Streptococcus thermophilus Sfi39 and Sfi12. Applied and Environmental Microbiology, 63, 3512-3518.
- Longo-Sorbello, G. S., Saydam, G., Banerjee, D., & Bertino, J. R. (2005). Cytotoxicity and cell growth assays. Cell biology, ed. JE Celis, N. Carter, K. Simons, JV Small, and T. Hunter, 315-324.
- Mohammadi, M. (2013). Probiotics and Cancer. Journal of Biology, 2(4), 202-209.
- Niki, E., Shimaski, H. & Mino, M. (1994). Antioxidantism-free radical and biological defense. Gakkai Syuppn Center, Tokyo, 3
- Pan, D., & Mei, X. (2010). Antioxidant activity of an exopolysaccharide purified from Lactococcus lactis subsp. lactis. Carbohydrate Polymers, 80(3), 908-914.
- Padmavathy, S. (2014). Evaluation of antioxidant activity of Gaultheria Fragratissima Wall. Asian Journal of Pharmaceutical Science & Technology, 4 (2), 65-67.
- Rochat, T., Miyoshi, A., Gratadoux, J. J., Duwat, P., Sourice, S., Azevedo, V., & Langella, P. (2005). High-level resistance to oxidative stress in Lactococcus lactis conferred by Bacillus subtilis catalase KatE. Microbiology, 151(9), 3011-3018.
- Rodrıguez, E., Calzada, J., Arqués, J. L., Rodrıguez, J. M., Nunez, M., & Medina, M. (2005). Antimicrobial activity of pediocin-producing Lactococcus lactis on Listeria monocytogenes, Staphylococcus aureus and Escherichia coli O157: H7 in cheese. International Dairy Journal, 15(1), 51-57.
- Rodrıguez, E., Calzada, J., Arqués, J. L., Rodrıguez, J. M., Nunez, M., & Medina, M. (2005). Antimicrobial activity of pediocin-producing Lactococcus lactis on Listeria monocytogenes, Staphylococcus aureus and Escherichia coli O157: H7 in cheese. International Dairy Journal, 15(1), 51-57.
- Roy, U., Batish, V. K., Grover, S., & Neelakantan, S. (1996). Production of antifungal substance by Lactococcus lactis subsp. lactis CHD-28.3. International journal of food microbiology, 32(1), 27-34.
- Shahidi, F., Janitha, P. K., & Wanasundara, P. D. (1992). Phenolic antioxidants. Critical Reviews in Food Science & Nutrition, 32(1), 67-103.
- Shalke, S. E. (2013). The application of probiotics in decrease cancer. Journal of Biomedical and Pharmaceutical Research, 2(3).
- Smith, M. A., Richey, G. P. P., Sayre, L. M., Anderson, V. E., Beal, M. F., & Kowall, N. (1996). Test for oxidative damage in Alzheimer’s. Nature, 382, 120-121.
- Squadrito, G. L., & Pryor, W. A. (1998). Oxidative chemistry of nitric oxide: the roles of superoxide, peroxynitrite, and carbon dioxide. Free Radical Biology and Medicine, 25(4), 392ity-403.
- Nguyen, T., Nguyen, D. (2014) Study on cytotoxicity activities in plant originated Lactobacillus rhamnosus. Wulfenia Journal, 21(5), 187-198.
- Nguyen, T., Doan, V., Ha, L., Nguyen, H. (2013). Molecular Cloning, Expression of minD Gene from Lactobacillus acidophilus VTCC-B-871 and Analyses to Identify Lactobacillus rhamnosus PN04 from Vietnam Hottuynia cordata Thunb. Indian Journal of Microbiology, 53(4): 385 – 390.
- Vincent, S.J.F., Faber, E.J., Neeser, J.R., Stingele, F., and Kamerling, J.P. (2001) Structure and properties of the exopolysaccharide produced by Streptococcus macedonicus Sc136. Glycobiology, 11, 131–139.
- Virtanen, T., Pihlanto, A., Akkanen, S., & Korhonen, H. (2007). Development of antioxidant activity in milk whey during fermentation with lactic acid bacteria. Journal of applied microbiology, 102(1), 106-115.
- Yen, G. C. & Chen, H. Y. (1995). Antioxidant activity of various tea extracts in relation to their antimutagenicity. Journal of Agricultural and Food Chemistry, 43(1), 27-32.








