Nasim Esnaashari Esfahani1*, Sosan Sadeghian2, Mohamad Razavi3, Mohsen Minaiyan4 and Erfane Afsari5
1Department of Orthodontics, Khorasgan (Isfahan) Branch, Islamic Azad University, Isfahan, Iran. 2Assistant Professor, Azad University of Khorasgan, Isfahan, Iran. 3Associated professor, Isfahan University of Medical Science, Esfahan, Iran. 4Assistant Professor, Isfahan University of Medical Science, Esfahan, Iran. 5Resident, Azad University of Khorasgan, Isfahan, Iran.
DOI : https://dx.doi.org/10.13005/bpj/414
Abstract
Simvastatin is widely used for lowering serum cholesterol, and also has protective effect on bone metabolism. The aim of this study was to evaluate Simvastatin effect on alveolar bone remodeling, root resorption and amount of tooth movement during orthodontic treatment in rats.In this animal study, 32 adult male rats were randomly divided in two groups. After application of a nickel titanium closed-coil spring with 60 g force between the maxillary central incisor and maxillary first molar, animals in the experimented group began receiving Simvastatin at a dose of 2.5 mg per kilogram per day for 17 days, and animals in the control group received normal saline. The distance between teeth was measured on 1 and 17 days. Two animals from each group were killed at 4, 7, and 17 days. Histo morphometric analyses of bone mineral appositional rate, percentage of root resorption area, and number of resorption lacunae of maxillary first molar mesiobuccal root were done. T-test independent were done for statistical analysis.The rats in experimental group showed significantly decrease of tooth movement (p<0.024). Mineral apposition rate at 7 and 17 days were increased significantly in experimental group(p<0.05).Percentage of root resorption and number of root resorption lacunae at 4,7 and 17 days were decreased significantly in experimental group(p<0.05).The result of this study showed Although Simvastatin might decrease root resorption related to orthodontic tooth movement, patients and clinicians should be informed about a possible decrease in the amount of tooth movement and a prolonged period of orthodontic treatment.
Keywords
Simvastatin; Bone Remodeling; Root Resorption; Tooth Movement
Download this article as:| Copy the following to cite this article: Esfahani N. E, Sadeghian S, Razavi M, Minaiyan M, Afsari E. The Effects of Simvastatin on Bone Remodeling, Tooth Movement and Root Resorption in Orthodontic Treatments. Biomed Pharmacol J 2013;6(2) |
| Copy the following to cite this URL: Esfahani N. E, Sadeghian S, Razavi M, Minaiyan M, Afsari E. The Effects of Simvastatin on Bone Remodeling, Tooth Movement and Root Resorption in Orthodontic Treatments. Biomed Pharmacol J 2013;6(2). Available from: http://biomedpharmajournal.org/?p=2720 |
Introduction
The factors affecting the rate of tooth movement, bone remodeling and root resorption while applying orthodontic forces is one of important challenges in orthodontic treatment. During orthodontic tooth movement, when the orthodontic forces are exerted on teeth and bones, extensive remodeling of bone, periodontal ligament, periosteum, cementum and sutures occurs. These changes represent the compatibility of periodontal ligament and its surrounding bone tissue [1]. Since orthodontic tooth movement is associated with mineral apposition, the factors influencing the remodeling process will affect the tooth movements. There are many studies on root resorption as a consequence of orthodontic treatment. It seems that this process is dependent on several factors. The factors associated with external root resorption can be divided into biological and mechanical factors. The effective mechanical factors include tooth movement, the movement type, amount and duration of orthodontic force [2]. The effective biological factors include genetic predisposition and systemic factors such as hormonal imbalances, root morphology, dental agenesis, and medications that the patient is receiving [3].
It is possible to use pharmacologic factors to influence tooth movement either for reducing (when anchorage strengthening is desirable) or increasing the movement [3]. The medications used by patients during orthodontic treatment can reach through the bloodstream to the tissues surrounding the teeth which are under mechanical stresses. These medications can also affect the local target cells [4]. Statins, 3-hydroxy-3- methyl glutaryl and inhibitor coenzyme, reductase, are of drugs which are widely used to lower blood serum cholesterol in adults due to their low prices [5].
Simvastatin is a chemical derivative of lovastatin. Many studies on animal models have proved the stimulatory effect of simvastatin on bone formation during its topical use by various carriers [5]. The results of an observational study on the people who used statins for lowering blood lipids showed increased bone density and less risk for bone fractures in these individuals [6]. Statins have been able to stimulate osteoblasts, thereby caused bone formation in vitro and human studies [7]. In a study on rats with periodontitis, the stimulatory effect of simvastatin on alveolar bone formation was observed [8].
However, there is no histological study on the effect of simvastatin on orthodontic tooth movement, bone remodeling and root resorption. The objective of the present study is to examine the effect of simvastatin on the rate of tooth movement, alveolar bone remodeling and root resorption during applying orthodontic force on rats.
Materials and Methods
Thirty two adult male rats were selected for this study. The age and weight of rats was about 8 to 10 weeks and 200-250 g, respectively. The samples were randomly divided into experimental and control groups. The initial weight of each animal was recorded following general anesthesia using ketamine hydrochloride 10% (40 mg/kg, Rumpun, Bayer Korea, Seoul). The distance between the tip of the maxillary central incisor edge and the edge of mesiobuccal cusp of the upper first molar on the left was measured using a digital caliper (1108-150, Insize Co, China).
Thereafter, the contact of the first and second maxillary molars was opened using a fissure turbine drill (flat end cylinder, 835, Teeskavan, Iran) and a 0.01” wire ligature (Ligature Ties 0.011, Orthotechnology, USA) with a nickel-titanium (Ni-Ti) closed coil spring (close coil springs with eyelets, Size 9F, G & H wire company) inside it was closed around the first molar and maxillary central incisor.
So that it would enter a 0.50 N force. The magnitude of force was selected according to several studies in which a force of 40 to 60 grams has been proposed to move the molar teeth of rats [9]. After placing the ligature wires, some composite (GRADIA DIRECT, GC, Japan) was cured on the wire of incisors region to prevent them from sliding.
According to the available literature on the simvastatin [10], it was found that its prescribed dose is 2.5 mg per kg of body weight per day. Given that rats weighing 200 to 250 g, a rat should receive 625 mg of drug daily. The volume of drug was below 1 ml given the situation of rats. A solution with a concentration of 0.06 mg of simvastatin was prepared such that it could be used for several times for all rats of experimental group for the entire study course.
The drug concentration was 600 µg/ml so that 1 ml of the solution was enough for a daily dose of a 240 g rat. A slightly less or slightly more than 1 ml of solution, (which was easy to determine by an insulin syringe) covered rats’ weight range easily.
Simvastatin was interperitoneally injected to the rats of experimental group for 17 days. The same amount of normal saline was daily injected to the rats of control group. The distance between the incisor and maxillary molar was recorded using a digital caliper on the first day after placing the spring. The measurement was recorded again on the seventeenth day after general anesthesia of rats. The difference between these two records is the tooth movement during the study period. Two animals from each group were killed on the fourth, seventh and seventeenth days after spring placement. The heads of samples were cut and stored in formalin 10%, then transferred to the laboratory.
In the laboratory, samples of the maxillary first molar with the surrounding bone were prepared. The mesiobuccal root of the maxillary first molar was selected for further study, because it has the largest root and yet it was the nearest region where the force applied. The samples were stabilized for 24 h in formalin 10% (Merck, Germany) and then placed in acid for another 24 h. Then the process was performed as follows. First, the sample was placed in 10% formalin for two hours. Then, it was placed in alcohol 75%, alcohol 85%, alcohol 95% and alcohol 100% for 1h, 1.25 h, 1.5 h and 2 h, respectively. After that, the sample was placed in Xylenol and paraffin with a temperature of 56 °C for 2h, respectively.
Thereafter, the tissues were molded. The molds were cut from coronal, middle and apical surfaces after mesiodistally with microtome (Leica, RM 2035, Germany). Three horizontal sections with a thickness of 5 µm were prepared. The as-prepared sections were placed on slides and marked with fluorescent. Then, hematoxylin and eosin staining was performed as follows:
The slides were placed in the oven with a temperature of 65 °C for 30 minutes.
The slides were placed in in three Xylenol containers for 15 minutes (each 5 minutes).
The samples were placed in alcohols with descending concentrations (100, 95, 85, 75 and 50) and finally placed in distilled water.
The slides were placed in hematoxylin (Merck, Germany) for 5 minutes.
The slides were rinsed in water and then were differentiated in acid alcohol.
The slides were placed in eosin (Merck, Germany) for 2 minutes.
The slides were placed in alcohols with ascending concentrations (75, 85, 95 and 100).
Finally slides were dried and mounted and were investigated using an optical microscope (Olympus BX – 51) with 100 and 400 times magnification. The mineral apposition rate (MAR) was determined by measuring the distance between the two fluorescent lines with the help of Nilu software (Nilu pathology image analyzer) and dividing it by the sampling time (mm/day). The number of root resorption lacunae (NRRL) in each cross section was calculated using the Nilu software. Then, the surface area of the whole root (SAWR) in each cross section was determined using the software, and the surface area of resorption lacunae (SARRL) in each section was calculated. The percentage of root resorption area (PRRA) was obtained using the following formula:
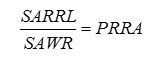
All these measurements were carried out by a pathologist for 2 times in 4 week intervals. The data was analyzed by SPSS and independent t-test.
Results
In this animal study, 32 adult male rats were randomly divided into 2 groups. After activation of a Ni-Ti closed coil spring between the first molar and maxillary central incisor with a force of 60 g, 2.5 mg/kg simvastatin was daily interperitoneally injected to the experimental group up to 17 days. The control group received normal saline. Tables 1 to 4 show the results of average rate of tooth movement, the average amount of bone formation, the mean percentage of root resorption and the number of resorption lacunae around the mesiobuccal root of maxillary first molar in both groups during the study period. The mean distance between the anterior maxillary first molar on the first day prior intervention in the control group was 12.88±0.44. It had no significant difference with the average tooth distance prior the intervention in the experimental group, 13.06±0.55 (p=0.377).
As can be seen, the paired t-test showed a significant difference between the mean first molar and the maxillary incisor in both groups before and after the intervention (P<0.5) (Table 1)
Table 1: The average distance between the maxillaryincisor and first molar during the study period in the two study groups
| Experimental group | Control group | (independent t-test)
P – value
|
|||
| Average | Standard deviation | Average | Standard deviation | ||
| Day 1 | 13.06 | 0.57 | 12.88 | 0.44 | 0.377 |
| Day 17 | 12.46 | 0.61 | 12.68 | 0.66 | 0.043 |
| P – value (T– (Paired-T) | 0001/0 P = | 0.003P value = | |||
Independent T-test showed that the tooth movement in the experimental group within 17 days was 0.59±0.33 which showed a significant difference with the mean value of the control group, 0.89±0.56 (P=0.024). The average bone formation in three different days including the fourth, seventh and seventeenth days was examined in both groups. Statistical analysis showed that the average bone formation on the fourth day in the experimental groups was lower than the control group. The observed difference was statistically significant (p=0.0001).
But the average bone formation on the seventeenth and seventh days was higher in the experimental group than the control group. This difference was statistically significant (Table 2).
Table 2: The average rate of bone formation on the fourth, seventh and seventeenth days in the two groups
| Experimental group | Control group | (independent t-test)
P – value Average |
||||
| Average | Standard deviation | Average | ||||
| Day 1 | 12/5 | 43/0 | 18/6 | 26/0 | 0001/0 | |
| Day 7 | 64/5 | 32/0 | 06/5 | 35/0 | 013/0 | |
| Day 17 | 21/6 | 27/0 | 28/3 | 18/0 | 0001/0 | |
The results of independent t-test on average root resorption showed that there is a significant difference between the two groups on fourth, seventh and seventeenth days of study. The root resorption in the experimental group was lower than the control group in all three time periods (P<0.05) (Table 3).
Table 3: The average root resorption on the fourth, seventh and seventeenth days in the two groups
| Experimental group | Control group | (independent t-test)
P – value Average Average |
||||
| Average | Standard deviation | Average | ||||
| Day 1 | 32.95 | 0.84 | 35.13 | 0.84 | 0.001 | |
| Day 7 | 24.38 | 0.67 | 26.21 | 1.50 | 0.025 | |
| Day 17 | 13.53 | 0.75 | 15.60 | 0.48 | 0.0001 | |
There was a significant statistically difference between the average number of resorption lacunae in the two groups on the fourth, seventh and seventeenth days. The number of resorption lacunae in the experimental group was lower than the control group in all three time periods (P<0.05) (Table 4).
Table 4: The average number of resorption lacunae on the fourth, seventh and seventeenth days in the two groups
| Experimental group | Control group | (independent t-test)
P – value Average Average Average |
||||
| Average | Standard deviation | Average | ||||
| Day 1 | 3.31 | 0.26 | 4.50 | 0.24 | 0.0001 | |
| Day 7 | 2.30 | 0.30 | 2.92 | 0.16 | 0.001 | |
| Day 17 | 1.66 | 0.18 | 2.33 | 0.37 | 0.003 | |
Discussion
The statins include: lovastatin, atorvastatin, follistatin, vastatin series and simvastatin. Simvastatin was selected in this study, because many studies have shown that it has the most stimulating bone formation ability compared to the other statins [5]. Furthermore, simvastatin is the most common form of statins for reducing blood lipids.
In this study, simvastatin was used in injectable form. The problem with its topical application in the mouth is mucous inflammation which is worsening at doses above 0.5 mg. A dose of 0.5 mg simvastatin is recommended to reduce inflammation in its topical application. This is a very low dose with limited side effects. Therefore, it can be said that the topical application of simvastatin will not cause any problem in areas such as the femur and calvarium which has not direct contact with the mucosa. But in the oral cavity, due to direct contact with the mucosa and inflammation, the dosage should be reduced. To solve this problem, it is better to use systemic form of simvastatin at higher doses [24].
In this study, a dose of 2.5 mg per kg of body weight selected [9]. At onset of study, the spacing of teeth were similar in both groups and showed no difference. Following injection of simvastatin and normal saline respectively in the experimental and control groups, 0.59 mm and 0.89 mm reduce in the teeth spacing between the maxillary incisor and molar was created, respectively. The experimental group showed a greater reduction in the rate of tooth movement and this difference was statistically significant. It means that simvastatin inhibits bone resorption or increases bone formation while applying orthodontic forces. Simvastatin has been able to effectively reduce tooth movement.
The rate of bone formation was used to determine the effect of simvastatin on the mineralization process while remodeling of alveolar bone. In the present study, the average rate of bone formation in the experimental group was significantly increased compared with the control group on the seventeenth and seventh days of the study. The observed difference was significant.
But on the fourth day, the rate of bone formation was higher in the control group than the experimental group. This difference was also significant. Simvastatin affect the bone metabolism in different ways. According to Takenaka, simvastatin differentiate osteoblasts and bone formation through affecting the release of VEGF [25]. The results of Mundy et al. showed that statins increase bone formation by osteoblasts through affecting the rising incidence of BMP – 2. At the same time, it prevents bone resorption through inhibiting the production of GTPase which is involved in activation of osteoclasts [7].
BMP – 2 is an osteogenic growth factor which is involved in differentiation of osteoblasts and mineralization [26]. According to Lin, simvastatin prevents formation of Cyr61 protein through inhibiting the effect of TNF-α on osteoblasts. Consequently, chemotaxis macrophages and bone resorption are reduced [23]. Sakoda found that simvastatin reduces the amount of IL-6 and 8 because of its anti-inflammatory effect. Since cytokine affects the activation of osteoclasts, it inhibits the inflammatory bone resorption [27].
Guanghong et al. found that simvastatin prevents tooth movement relapse after orthodontic treatment by affecting the osteoprotegrin (OPG) to RANKL ratio in the periodontal tissues and preventing resorption activity of osteoclasts. OPG protects bone against osteoclasts and its expression increase will reduce bone resorption [10].
The results of the present study confirm all influencing trends of simvastatin on bone formation. Perhaps the reason for the difference on the fourth days can be expressed as follows. Wang et al. examined the effect of simvastatin on the grafts used to repair bone defects. They found that statin affects VEGF and BMP-2 increase on the third and fourth days after surgery, respectively. All of these were on a day earlier than the group in which only graft was used [19]. Therefore, perhaps due to increased bone formation on the seventeenth and seventh days, the effect of simvastatin on generation of proteins and cytokines that affect bone formation occurs with a delay of a few days.
The percentage of root resorption areas and the number of resorption lacunae within three days were also investigated. It was found that in all three days, the percentage of root resorption areas and the number of resorption lacunae in the experimental group were significantly lower than the control group. It is clear that while applying orthodontic force on the pressure region, cytokines such as prostaglandin E2, interleukin 1 and 6 and TNf α are released from PDL cells. All of these factors leads to inflammation and RANKL increase, thereby osteoclasts are activated and root and alveolar bone resorption occur [24]. The studies conducted by Low and Al-Qawasmi [29] showed that the relationship between OPG and RANKL will affect the root resorption. Given that simvastatin increases OPG to RANKL ratio in periodontal tissues, it can be a factor in preventing root resorption.
According to histologic study of Mundy et al., the reduction in the number and activity of osteoclasts was observed after taking simvastatin [7]. According to Sakoda et al., simvastatin reduces inflammatory activity through reduction of interleukin 6 and 8 [27]. It seems that it can prevent cellular reactions leading to hyalinization tissue removal and resorption of the superficial parts of cementum. Pursuant to this, the delay in the onset and rate of tooth movement occurs. The clinical results of the present study showed the same finding. Furthermore, according to Okamoto et al., the use of simvastatin increases dentin sialophosphoprotein (DSPP) gene expression and osteocalcin. Both of these result in differentiation of odontoblasts and generation of hard dental tissues [30]. So it seems that simvastatin can reduce the amount of root resorption during orthodontic treatment through mentioned processes. Overall, understanding the influence of simvastatin on the different paths of growth and generation of bone allows the use of this medication and similar drugs in therapeutic strategies to affect bone quality and quantity.
Conclusion
The results of the present study indicate that simvastatin inhibits bone resorption and increases bone formation. At the same time, it also reduces the amount of root resorption. Therefore, clinicians and patients should be aware of tooth movement reduction and prolonged orthodontic treatment while using simvastatin. However, the topical use of simvastatin may be employed in the future for strengthening anchorage and preventing movement of dental units. In this study, the limitations include limited number of samples, and harmless maintain of devices in the mouth of rats.
It is recommended to investigate the immunohistochemistry of simvastatin on the rate of tooth movement, bone remodeling and root resorption in orthodontic patients. The radiological study of the effects of simvastatin on tooth movement, bone remodeling and root resorption in orthodontic patients, the effect of topical simvastatin on orthodontic patients to strengthen anchorage and root resorption reduction and the effect of simvastatin on the improvement of root resorption lesions are also recommended.
References
- TM G. Orthodontics:Current principle and techniques. ed t, editor. Mosbyco: Elsivier; 2005.
- A A. Biomechanical aspects of external root resorption in orthodontic therapy. Med Oral Patol Oral Cir Bucal Dec. 2007 12:E610-3.
- Profit W HW. Contemporary orthodontics. ed t, editor. Mosbyco: Elsivier; 2007.
- Krishnon V DZ. Biological mechanisms of tooth movement. ed s, editor.: wiley-Blackwel; 2009.
- JB P. The use of Simvastatin in bone regeneration. Med Oral Patol Oral Cir Bucal Dec. 2009;14:485-8.
- Edwards CJ HD, Spector TD. Oral statins and increased bone mineral density in postmenopausal women. Lancet 2000;24:2218- 9.
- Mundy G GR, Harris S, ChanJ, Chen D, Rossini G, Boyce B, Zhao M, Gutierrez G. Stimulation of bone formation in vitro and in rodents by statins. Science 1999;286:1946–9.
- Seto H OH, Tokunaga K, Hama H, Horibe M, Nagata T. Topical administration of simvastatin recovers alveolar bone loss in rats. J Periodontal Res. 2008;43:261-7.
- Choi J BS, Lee J. Effect of clodronate on early alveolar bone remodeling and root resorption related to orthodpntic forces:A histomorphometric analysis. A merican Journal of Orthodontics and Dentofacial Orthopedics. 2010;138:548e1-e7.
- Guanghong H YC, Jianhua H. Effects of Simvastatin on relaps and remodeling of periodontal tissues after tooth movement in rats. American journal of orthodontics. 2010550e1-e6.
- Chay SH RA, Itthagarun A. Ultrastructural identification of cells involved in the healing of intramembranous bone grafts in both the presence and absence of demineralised intramembranous bone matrix. Aust Orthod J. 2000 16:88-97.
- Maeda T MA, Kawane T, Horiuchi N. Simvastatin promotes osteoblast differentiation and mineralization in MC3T3- E1 cells. Biochem Biophys Res Commun. 2001;280 874 – 7.
- Skoglund B FC, Aspenberg P. Simvastatin improves fracture healing in mice. J Bone Miner Re. 2002 17:2004-8.
- Wong RW RA. Statin collagen grafts used to repair defects in the parietal bone of rabbits. Br J Oral Maxillofac Surg. 2003;41:244–8.
- Hatano H MA, Bolander ME,Sarkar G. Stain stimulates bone morphogenic protein-2,aggrecan,and type 2 collagen expression and proteoglycan synthesis in rat chondrocytes. J orthop Sci 2003;8:842-8.
- Thunyakitpisal PD CR. Simvastatin,an HMG-CoA reductase inhibitor,reduced the expression of matrix metalloproteinase -9 in osteoblastic cells and HT1080 fibrosarcoma cells. J Pharmacol Sc. 2004;94:403-9.
- Ayukawa Y OA, Koyano K. Simvastatin promotes osteo-genesis around titanium implants. Clin Oral Implants Res. 2004;15:346–50.
- Viereck V GC, Blaschke S. Atrovastatin stimulates the production of osteoprotegerin by human osteoblastic. J Cell Biochem. 2005;15:1244-53.
- Wong RW RA. Histologic and ultrastructural study on statin graft inrabbit skulls. J Oral Maxillofac Surg. 2005;63:1515–21.
- Yazawa H ZB, Asami Y, Bernimoulin JP. Simvastatin promotes cell metabolism, proliferation, and osteoblastic differentiation in human periodontal ligament cells. J Periodontol. 2005 76:295-302.
- Hatzigeorgiou C JJ. Hydroxymethylglutaryl-coenzyme A reductase inhibitors and osteoporosis: a meta-analysis. Osteoporos Int 2005;16:990-8.
- Ruse-Gaspa S NX, Enjuanes A. simvastatin and atorvastatin enhance gene expression of collagn yype 1 and osteocalcin in primary human osteoblasts and MG-63 cultures. J Cell Biochem. 20071430-8.
- Lin SK KS, Lee YL. Simvastatin as a Novel Strategy To Alleviate PeriapicalLesions. Journal of Endodontics. 2009;35:657-62.
- Yamaguchi M Kt, kanekawa M, Aihara N. Neuropeptid stimulate production of interleukin-1 beta,inter leukin-6,and tumor necrosis factor-alpha in human dental pulp cells. Inflammation Reaserch. 2004;5:199-204.
- Takenaka M HK, Tanabe K, Akamatsu S, Dohi S, Matsuno H. Simvastatin stimulates VEGF release via p44/p42 MAP kinase in vascular smooth muscle cells. Biochem Biophys Res Commun. 2003;301:198-203.
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383–9.
- Sakoda K YM, Negishi Y, Liao JK, Node K, Izumi Y. Simvastatin decreases IL-6 and IL-8 production in epithelial cells. J Dent Res. 2006;85:520-3.
- LOW E ZH, Kharbanda OP, Darendeliler MA. Expression of mRNA for osteoprotegerin and receptor activator of nuclear factor kappa beta ligand (RANKL)during root resorption induced by the application of heavy orthodontic forces on rat molars. American Journal Of orthodontics and Dentofacial Orthopedics. 2005;128:497-503.
- Al-Qawasmi Ra HJ, Everett ET, Flury L. Genetic predisposition to external root resorption in orthodontic patients:linkage of chromosome-18 marker. Journal of Dental research. 2003;82:356-60.
- Okamoto y SW, One M. Simvastatin induces the odontogenic differentiation of human dental pulp stem cells in vitro and in vivo. J Endod. 2009;35:367







