Manuscript accepted on :
Published online on: 16-12-2015
Plagiarism Check: Yes
Ndubuisi N. Nwobodo
Department of Pharmacology and Therapeutics, Faculty of Clinical Medicine, Ebonyi State University, Abakaliki, Nigeria.
DOI : https://dx.doi.org/10.13005/bpj/397
Abstract
The pleiotropic effects of statins suggest that their therapeutic benefits may probably extend beyond cholesterol lowering in cardiovascular disease to organ transplantation and other disease conditions, particularly autoimmune disease. This is attributable to their modulating influence on immune mechanisms mediated by inhibition of prenylated proteins. Isoprenoid intermediates generated by the mevalonate pathway are involved in post translational prenylation of signal transduction proteins. Reduction in allograft rejection is linked to immunomodulatory function of statins; graft rejection been enhanced by down regulation of pro-inflammatory T-helper 1 cells. Conclusively, the therapeutic potential of statins has been proven in organ transplantation and other disease conditions due to their potent immunomodulatory characteristic.
Keywords
Autoimmune disease; Immunomodulation; Organ Transplantation; Protein prenylation; Statins; Therapeutic benefit
Download this article as:| Copy the following to cite this article: Nwobodo N. N. Statin Immunomodulation: Role in Transplantation and Disease Conditions. Biomed Pharmacol J 2013;6(2) |
| Copy the following to cite this URL: Nwobodo N. N. Statin Immunomodulation: Role in Transplantation and Disease Conditions. Biomed Pharmacol J 2013;6(2). Available from: http://biomedpharmajournal.org/?p=2660 |
Introduction
Clinical and experimental data support the protective role of statins against the rejection of organ transplants. Statins have been shown to be the most potent and effective drug for serum cholesterol lowering1. The therapeutic potential of statins as immunosuppressive agent has been demonstrated by clinical cases of transplant survival. Statins have been shown to offer protection against rejection in heart transplants2 and found to be beneficial in pulmonary and renal transplantation3-6. Considerable interest has been shown in the therapeutic potential of statins in autoimmune disease conditions7-9 such as rheumatoid arthritis, multiple sclerosis, encephalomyelitis; to conditions such as chronic kidney disease, respiratory disease and sepsis. This paper highlights the immunological response mediated by statins in organ transplantation and other disease conditions with particular reference to autoimmune diseases.
Statin Immunomodulation and Organ Transplantation
The prevalence of hypercholesterolemia in heart transplant patients necessitates the administration of statins to prevent transplant atherosclerosis. A decrease in graft atherosclerosis is consistent with immunomodulatory function of statins. The prevention of graft rejection can be enhanced by a shift towards T-helper 2 cell differentiation or down regulation of pro-inflammatory T-helper 1 cells. A study revealed diminution in host inflammatory cell recruitment without reduced lipid levels and attenuation of transplant graft arterial disease10. It has been shown that the inhibition of interferon-γ (IFN-γ) induced expression of MHC class II on antigen presenting cells is the major underlying mechanism of immunosuppression in organ transplantation11. Graft rejection after organ transplantion is influenced by MHC class II molecules which have direct effect on the control of immune responses.
The LFA1/ICAM1 interaction mediated by T-cell co-stimulation via statin modulation has an important role in pathophysiology of transplant rejection12. Nonetheless, in organ transplant recipients, the combination of an immunosuppressive effect with powerful cholesterol-lowering action would be particularly desirable. A study has noted that in many organ transplant recipients, there was a high incidence of hypercholesterolemia13. It was revealed that in patients, with pre-existing atherosclerosis, the highest incidence of post-transplantation hypercholesterolemia given as 80% was found, surprisingly in heart transplant patients. It is remarkable to note that hypercholesterolemia independent of transplantation has been linked to standard immunosuppressive agents (cyclosporine and corticosteroids) administered to transplant recipients. Significant decline in rejection rates has been reported in statin-treated patients following renal transplantation4,5. Data from a systematic review of 13 randomized controlled intervention trials indicated that the use of statins improved cardiovascular risk and reduced clinical cardiac events, although the risk of acute rejection after renal transplantation was not reduced14.
A beneficial effect on chronic allograft nephropathy was reported by retrospective study aimed at determining the impact of statins on graft outcome in the first year following transplantation6. The study further lent credence to the fact that improved functional and histological outcome in the first year results following early introduction of statins, post-transplantation; which impacts on the pathology of chronic allograft rejection significantly.
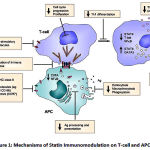 |
Figure 1: Mechanisms of Statin Immunomodulation on T-cell and APC Functions
|
The interferon-γ induced expression of MHC class II under the influence of MHC-II transactivating factor and expression of co-stimulatory molecules by APCs are inhibited by statins. Statins inhibit Signal Transducer and Activator of Transcription 4 (STAT4) necessary for T helper1 cell differentiation, while enhancing the effect of STAT6 and GATA3 responsible for that of T helper2 cell. Modified and Adapted from-Greenwood J., Steinman L. and Zamvil S.S. Statin therapy and autoimmune disease: from protein prenylation to immunomodulation. Nature. 6: 358-370 (2006).
Statin Immunomodulation in Disease Conditions
Statins have been shown to exhibit therapeutic potential in immunological disorders due to their potent immunomodulatory characteristics. Chronic and relapsing paralysis in multiple sclerosis was not only prevented by atorvastatin but reversed15,16. Beneficial effects of statins in multiple sclerosis and rheumatoid arthritis have been reported17,18. A study reported that lovastatin treatment over a 12 month period resulted in a decrease in the mean number of gadolinium enhancing lesions but no accompanying reduction in the expanded disability status scale (EDSS) score, in a purely observational study of seven patients with relapsing-remitting multiple sclerosis19. Although, it is well known that statins reduce cardiovascular risk in individuals with normal renal function20,21; there are some encouraging data indicative that this effect is also seen in individuals with chronic kidney disease. Pravastatin reduced rates of cardiovascular events in patients with or at high risk of coronary disease plus the combination of diabetes and stage 2 or early stage 3 chronic kidney disease, in a sub-study of three randomized clinical trials: Cholesterol and Recurrent Events (CARE), West of Scotland Coronary Prevention Study (WOSCOPS) and Long-Term Intervention with Pravstatin in Ischemic Disease (LIPID)22. Results from clinical trial involving rosuvastatin indicate that the drug might arrest the progression of kidney disease23. The fact that pravastatin moderately reduced the rate of kidney function decline was further revealed by pooled analysis of data from WOSCOPS, CARE, and LIPID trials. Analysis of the GREeK Atorvastatin and coronary heart disease evaluation (GREACE) study showed that atorvastatin prevented decline of creatinine clearance and improved renal function24. The role of statins in decreasing blood pressure has been demonstrated in a model of normocholestrolemic spontaneously hypertensive rat25. Nitric oxide production, a crucial mediator of vascular homeostasis and blood flow is enhanced by statin26,27. It has been shown in humans that hypercholesterolemia results in blood pressure rise due to angiotensin II enhancement induced by AT1 receptor overexpression28. Atorvastatin decreased blood pressure and protected against end-organ damage in an experimental model of hypertensive Dahl salt-sensitive rats prone to cardiovascular, renal and endothelial damage when fed on high salt diets29. A study revealed that the addition of pravastatin to the treatment regimen of hypertensive proteinuric patients treated with antihypertensives decreased the severity of proteinuria and this was attributable to a decline in urine endothelin I levels30. The same pravastatin was reported to lower diastole, systole and pulse pressures in a randomized placebo controlled study involving individuals with untreated hypertension and moderate hypercholesterolemia31. There are still obvious differences amongst various statins with regards to their pharmacokinetics, clinical efficacy and potency. It is unclear which statins will provide the best therapeutic benefit in a given disease condition. However, it remains controversial whether the benefits of statins demonstrated in animal studies translated to humans are mediated by the same mechanism.
In conclusion, statins possess proven therapeutic benefits in organ transplantation and other disease conditions attributable to their immunomodulatory properties. Notwithstanding, the need for large scale and long term clinical trials to ascertain the need for widespread recommendation and use of statins in autoimmune and other disease conditions can never be overemphasized.
References
- LaRosa J.C. What do statins tell us? Am. Heart J. 144(Suppl.): S21-S26 (2002).
- Kobashigawa J.A., Katznelson S., Laks H., Johnson J.A., Yeatman L., Wang X.M., Chia D., Terasaki P.I., Sabad A. and Cogert G.A. Effect of pravastatin on outcomes after cardiac transplantation. N. Engl. J. Med. 333(10): 621-627 (1995).
- Johnson B.A., Iacono A.T., Zeeri A., McCurry K.R. and Duncan S.R. Statin use is associated with improved function and survival of lung allografts. Am. J. Resp. Crit. Care Med. 167(9): 1271-1278 (2003).
- Katznelson S., Wilkinson A.H., Kobashigawa J.A., Wang X.M., Chia D., Ozawa , Zhong H.P., Hirata M., Cohen A.H., Teraski P.I. The effect of pravastatin on acute rejection after kidney transplantation-a pilot study. Transplantation. 61(10): 1469-1474 (1996).
- Tuncer M., Suleymanlar G., Ersoy F.F. and Yakupoqlu G. Comparison of the effects of simvastatin and pravastatin on acute rejection episodes in renal transplant patients. Transplant Proc. 32(3): 622-625 (2000).
- Masterson R., Hewitson T., Leikis M., Walker R., Cohney S. and Becker G. Impact of statin treatment on 1-year functional and histologic renal allograft outcome. Transplantation. 80(3): 332-338 (2005).
- Zamvil S.S. and Steinman L. Cholesterol-lowering statins possess anti-inflammatory activity that might be useful for treatment of multiple sclerosis. Neurology. 59: 970-971 (2002).
- Gurevich V.S., Shovman O., Slutzky L., Meroni P.L. and Shoenfield Y. Statins and autoimmune diseases. Autoimmun. Rev. 4: 123-129.
- Liao J.K. Isoprenoids as mediators of the biological effects of statins. J. Clin. Invest.110: 285-288 (2002).
- Shimizu K., Aikawa M., Takayama K., Libby P. and Mitchelle R.N. Direct anti-inflammatory mechanisms contribute to attenuation of experimental allograft arteriosclerosis by statins. Circulation. 108(17): 2113-2120 (2003).
- Kwak B., Mulhaupt F., Myit S. and Mach F. Statins as newly recognized type of immunomodulator. Nat. Med. 6(12): 1399-1402 (2000).
- Weitz-Schmidt G., Welzenbach K., Brinkman V., Kamata T., Kallen J., Bruns C., Cottens S., Takada Y. and Hommer U. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat. Med. 7: 687-692.
- Fellstrom B. Impact and management of hyperlipidemia post-transplantation. Transplantation. 70(Suppl.11): S51-S57 (2000).
- Lentine K.L. and Brennan D.C. Statin use after renal transplantation: a systematic quality review of trial-based evidence. Nephrol. Dial. Transplant. 19: 2378-2386 (2004).
- Aktas O., Waiczies S., Smorodchneko A., Dorr J., Seeger B., Prozorovski T., Sallach R., Endres M., Brocke S., Nitsch R. and Zipp F. Treatment of relapsing paralysis in experimental encephalomyelitis by targeting Th1 cells through atorvastatin. J. Exp. Med. 197(6): 725-733 (2003).
- Youssef S., Stuve O., Patarroyo J.C., Ruiz P.J., Radosevich J.L., Hur E.M., Bravo M., Sobel R.A., Steinman L. and Zamvil S.S. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 420(6911): 78-84 (2002).
- Abud-Mendoza C., de la Fuente H., Cuevas-Orta E., Baranda L., Cruz Rizo J. and Gonzalez-Amaro R. Therapy with statins in patients with refractory rheumatic diseases: a preliminary study. Lupus. 12(8): 607-611 (2003).
- Vollmer T., Key L., Durkalski V., Tyor W., Corboy J., Markovic-Plese S., Preiningerova J., Rizzo M. and Singh I. Oral simvastatin treatment in relapsing-remitting multiple sclerosis. Lancet. 363(9421): 1607-1608 (2004).
- Sena A., Pedrosa R. and Morais M.G. Therapeutic potential of lovastatin in multiple sclerosis. J. Neurol. 250: 754-755 (2003).
- Serruys P.W., de Feyter P., Macaya C., Kokott N., Puel J., Vrolix M., Branzi A., Bertolami C., Jackson G., Strauss B. and Meier B. Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. JAMA. 287(24): 3215-3222 (2002).
- Shepherd J., Cobbe S.M., Ford I., Isles C.G., Lorimer A.R., MacFarlane P.W., McKillop J.H. and Packard C.J. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia: West of Scotland Coronary Prevention Study Group. N. Engl. J. Med. 333(20): 1301-1307 (1995).
- Tonelli M., Keech A., Shepherd J., Sacks F., Tonkin A., Packard C., Pfeffer M., Simes J., Isles C., Furberg C., West M., Curhan G. Effect of pravastatin in people with diabetes and chronic kidney disease. J. Am. Soc. Nephrol. 16(12): 3748-3754 (2005).
- Vidt D.G., Cressman M.D., Harris S., Pears J.S. and Hutchinson H.G. Rosuvastatin-induced arrest in progression of renal disease. Cardiology. 102(1): 52-60 (2004).
- Athyros V.G., Mikhailidis D.P., Papageorgiou A.A., Symeonidis A.N., Pehlivanidis A.N., Bouloukos V.I. and Elisaf M. The effect of statins versus untreated dyslipidemia on renal function in patients with coronary heart disease. A subgroup analysis of the Greek atorvastatin and coronary heart disease evaluation(GREACE) study. J Clin. Pathol. 57(7): 728-734 (2004).
- Wassmann S., Laufs U., Baumer A.T., Muller K., Ahlbory K., Linz W., Itter G., Rosen R., Bohm M. and Nickenig G. HMG-CoA reductase inhibitors improve endothelial dysfunction in normocholesterolemic hyprtension via reduced production of reactive oxygen species. Hypertension. 37: 1450-1457 (2001).
- Laufs U., Fata L., Plutzky J. and Liao J.K. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 97: 1129-1135 (1998).
- Hernandez-Perera O., Perez-Sala D., Navarro-Antolin J., Sanchez Pascuala R., Hernandez G., Diaz C. and Lamas S. Effects of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors, atorvastatin and simvastatin, on the expression of endothelin-1 and endothelial nitric oxide synthase in vascular endothelial cells. J. Clin. Invest. 101(12): 2711-2719 (1998).
- Nickenig G., Baumer A.T., Yemur Y., Kebben D., Jockenhovel F. and Bohm M. Statin-sensitive dysregulated AT1 receptor function and density in hypercholesterolemic men. Circulation. 100(21): 2131-2134 (1999).
- Zhou M.S., Jaimes E.A. and Raij L. Atorvastatin prevents end-organ injury in salt-sensitive hypertension: role of eNOS and oxidant stress. Hypertension. 44(2): 186-190 (2004).
- Lee T.M., Lin M.S., Tsai C.H. and Chang N.C. Add-on and withdrawal effect of pravastatin on proteinuria in hypertensive patients treated with AT receptor blockers. Kidney Int. 68(2): 779-787 (2005).
- Glorioso N., Troffa C., Filigheddu F., Dettori F., Soro A., Parpaglia P.P., Collatina S. and Pahor M. Effect of the HMG-CoA reductase inhibitors on blood pressure in patients with essential hypertension and primary hypercholesterolemia (1999). Hypertension. 34: 1281-1286 (1999).







