Udaya Sakthi M, Josephine Ritashinita Lobo F. and Kiran B. Uppuluri*
Department of Pharmacy, School of Chemical and Biotechnology, SASTRA University, Thanjavur- 613 401, India.
DOI : https://dx.doi.org/10.13005/bpj/425
Abstract
Self-emulsifying drug delivery systems (SNEDDS) are highly in quest due to their innumerable benefits like better portability, improved stability and higher drug loading, coupled with ease and economy of their production. SNEDDS are used to improve the solubility of high lipophilic drugs. SNEDDS are improving the oral bioavailability of limited water-soluble drug compounds. These SNEDDS are isotrophic mixtures of oil, surfactant, co-surfactant and drug that form oil in water emulsion in aqueous environment under gentle agitation. This paper presents an overview of the design, preparation and characterization, effects on the bioavailability of various drugs and various literature reports on diverse types of self-emulsifying formulations.
Keywords
SNEDDS; Poorly water soluble drugs; Formulation; Characterization
Download this article as:| Copy the following to cite this article: Sakthi U. M, Lobo F. J. R, Uppuluri K. B. Self Nano Emulsifying Drug Delivery Systems for Oral Delivery of Hydrophobic Drugs. Biomed Pharmacol J 2013;6(2) |
| Copy the following to cite this URL: Sakthi U. M, Lobo F. J. R, Uppuluri K. B. Self Nano Emulsifying Drug Delivery Systems for Oral Delivery of Hydrophobic Drugs. Biomed Pharmacol J 2013;6(2). Available from: http://biomedpharmajournal.org/?p=2753 |
Introduction
Most of the drugs which are recently discovered are poorly water soluble and the oral delivery of these drugs have become a major problem. Drugs of Class II or IV, as per Biopharmaceutical classification system (BCS) exhibit poor aqueous solubility. The oral delivery of these drugs are affected by low bioavailability, erratic absorption, inter and intra subject variability and lack of dose solubility. Another important factor that affects the oral bioavailability is poor gastro intestinal permeability. BCS class III drugs show poor membrane permeability1. Hence to enhance their solubility and to increase its oral bioavailability lipid based formulations have emerged as a boon.
Lipid based formulations such as self nano emulsified delivery systems (SNEDDS) are said to increase the absorption of the lipophilic drugs. SNEDDS are isotropic mixtures of oil, surfactant, co-surfactant and drug that form oil in water emulsion in aqueous environment under gentle agitation. This forms a good mode for delivering poorly soluble drugs orally by increasing their bioavailability and stability. They offer large interfacial area between the oil and GIT fluids and enhance the rate of absorption of the drugs2. The unique characteristics of Self emulsifying drug delivery systems are given in Fig.1.
 |
Figure 1
|
These SNEDDS are given in the form of soft or hard gelatin capsules. They reach the gastro intestinal tract and the GI motility of the stomach provides the agitation for self-emulsification. Because of this self-emulsification the drug is given as small droplets with size less than 5µm for improved solubility. After administering orally, lingual and pancreatic lipases act on the oily phase of the SNEDDS that result in the formation of emulsified mono-glycerides, di-glycerides and fatty acids. This in the presence of bile acids leads to the formation of intestinal mixed micelles. When these mixed micelles pass through the enterocytes, it leads to the formation of chylomicrons. These drain the drug into the lymphatic vessels and not in the blood vessels thus bypassing the first pass effect. Thus the oral bioavailability gets increased3.
Components Of Snedds
It is very important to select and optimize the quantities of the SNEDDS components. Because the components of SNEDDS and their concentrations will influence the various characteristics of nano emulsions, such as droplet size, polydispersity index, self-nano emulsification time and in vitro drug release. In general, the selection of the components based on their ability to solubilize the drug of interest and also on their ability to form spontaneous emulsions/nanoemulsions. The various components of SNEDDS used are shown in table 1.
Table 1: Components of SNEDDS
| Component | Examples | |
| Lipids and oils
|
Fatty acids | Palmitic acid, Stearic acid, Oleic acid |
| Fatty acid esters | Glyceryl monooleate [Capmul1 GMO,Imwitor1 948, Peceol1], Glyceryl monostearate [Capmul1 GMS-50, Imwitor1 191], Glyceryl monolinoleate [MaisineTM 35-1], Glyceryl palmito stearate [Precirol1 ATO 5], Glyceryl behenate [Compritol1 888 ATO] , Ascorbyl palmitate, Medium chain mono- and diglycerides [Capmul1 MCM], Medium chain triglycerides [Labrafac1 CC Miglyol1 810 and 812], Glyceryl dilaurate, Propyleneglycol monolaurate [Lauroglycol1 FCC, Capmul1 PG-12] | |
| Propyleneglycol esters | Propyleneglycol monolcaprylate [Capryol1 90, Capmul1 PG-8], Propylene glycol dicaprylocaprate [Labrafac1 PG] | |
| Miscellaneous | Stearyl alcohol, Phospholipids, Bees Wax, Vitamin E | |
| Surfactants/ stabilizers | Caprylocaproyl polyoxyl-8-glycerides [Labrasol1], Polyoxyethylene sorbitan fatty acid esters [Tween1], Polyoxyethylene castor oil derivatives [Cremophor1, Lipocol1], Polyvinyl alcohol, Sorbitan esters [Span1], Tocopherol polyethylene glycol, succinate (TPGS)
Macrogol fatty acid glycerides [Gelucire1 44/14, Gelucire1 50/13], Hydroxyl propyl methyl cellulose, Poloxamer, Phospholipids and PEGylated phospholipids, Polyvinyl pyrrolidone, Bile acids (sodium deoxycholate), Cellulose derivatives, Polyglyceryl-3 dioleate [Plurol1, Oleique1 CC497] |
|
| Co-surfactants /co-solubilizers /co-stabilizers | Propylene glycol, Glycofurol, Phospholipids, Oleoyl/linoleoyl polyoxyl-6-glycerides [Labrafil1], Polyethylene glycol, Triacetin, Ethanol, Diethylene glycol monoethyl ether [Transcutol1 HP] | |
Oil phase
SNEDDS are isotropic mixtures of oil, surfactants and co surfactants that form fine oil-in-water nano emulsions upon mild agitation, followed by injection into aqueous media, such as GI fluids. The oil phase should be selected appropriately for the drug as its properties govern the solubility of the drug. This also have effect on droplet size of the emulsion and the rate at which emulsification takes place. Small droplet size is needed for good emulsification to occur. Solubility can be assessed by HPLC after overnight shaking of drug with specific quantities of different oils with the drug. Hence most suitable oil can be selected by characterizing many oils. Mixture of oils can also be used for getting optimum solubility of the drug.
Surfactant
Surfactant has great effect on the emulsification process, nano-emulsifying region and the droplet size. Some of the properties that are to be considered are HLB in oil, viscosity and affinity to oil phase. Surfactants are screened based on the emulsifying ability and this is done by mixing oil and surfactants under warming conditions and then diluted with deionized water to form isotropic mixtures. Once equilibrium obtained, percentage transmittance can be checked using spectrophotometer and droplet size, polydispersity index were measured by zeta sizer3. Concentration of the surfactant influences the droplet size of the emulsion. Irritation in GI mucosa and skin may be caused by these surfactants at higher concentrations. The acceptability of the surfactant for the specific route of administration should also be considered as this may cause some adverse effects. They can be used alone or in combination.
Co surfactants
Co-surfactants are used to improve emulsification of the surfactant. They are also screened by mixing various co-surfactants with selected surfactant and oily phase under warming conditions and then diluted with water to form isotropic mixtures. This will allows attaining equilibrium and then percentage transmittance, droplet size and polydispersity index must be measured.
Construction of pseudo-ternary phase diagrams
Various ratios of oil phase to surfactant and co-surfactant from 1:9 and 9:1 can be selected to sense the region of self-emulsification by constructing pseudo-ternary phase diagrams. In each step 5ml amount of water is added and titrated to the homogeneous mixture of oil and surfactant and visually observed. The transition from transparency to turbidity was determined by weight measurements. The boundaries of nano-emulsion were determined by constructing phase diagrams by using software 5.
Nature of the aqueous phase
The nature of the aqueous phase where the SNEDDS is going to be delivered also plays an important role as they influence the droplet size and stability of the formulation. So while designing SNEDDS, evaluation of the characteristics of SNEDDS in different aqueous phases at different pH and electrolyte concentration is mandatory. The evaluation should be done not only in water but also in ringer’s solution, simulated intestinal fluid (pH 6.8), simulated gastric fluid (pH 1.2) and in phosphate buffered saline.
Compatibility studies
Compatibility between the drug and the excipients should be studied using Differential scanning calorimetry and Fourier Transforms Infra-red spectroscopy 6.
Preparation of SNEDDS
From the pseudo-ternary phase diagrams, the concentration of oil and surfactant/co-surfactant concentrations could be determined and then the formulations can be prepared. The drug and the oil- surfactant mixture can be mixed together using vortex mixer at ambient temperature 7.
Preparation Of Solid-Snedds
Solid SNEDDS can be prepared by using Buchi 190 nozzle-type mini-spray dryer. Carriers are used to prepare solid- SNEDDS by suspending in 100ml of solvent. Hydrophobic carriers can be suspended in 100ml of ethanol and hydrophilic carriers can be suspended in 100ml of water. To these solutions, the liquid SNEDDS will be added with continuous mixing at room temperature to obtain good emulsions (Fig 2). Using peristaltic pump, the solution can be delivered through the nozzle (0.7 mm diameter) at a flow rate of 5ml/min and it will be spray dried at an inlet temperatures of 100 and 60 ͦ C and outlet temperature of 80 and 40 ͦ C. The direction of the air flow and the direction of the sprayed product should be the same. Some of the examples of solid carriers are Silicon dioxide and magnesium stearate which are hydrophobic, Polyvinyl alcohol (PVA), Sodium carboxy methyl cellulose (Na-CMC) and Hydroxypropyl-β-cyclodextrantrin (HP-β-CD) which are hydrophilic. Silicon dioxide is the mostly used solid carrier which gives efficient increase in the dissolution and oral bioavailability of the drug.8
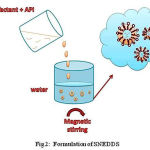 |
Figure 2
|
Characterization of the Snedds
Various parameters like droplet size and polydispersity index, colloidal stability and self-nanoemulsification time of the SNEDDS as a function of extent of dilution and variation in the pH/electrolyte content of aqueous phase need to be studied to characterize the final SNEDDS. To get an idea about the colloidal stability the zeta potential of the SNEDDS should be evaluated. The nanoemulsion droplets morphology can be studied by transmission electron microscopy. Various dissolution media have to be adopted to study the invitro dissolution profile of SNEDDS. The chemical stability of the drug in SNEDDS should be evaluated by carrying out long-term storage stability studies9.
Table 2: Grades of Nano emulsions based on visual appearance.
| S.NO | OBSERVATION | VISUAL ASPECT | GRADE |
| 1. | Nano emulsion formation in less than 30s, which is clear and transparent, high spreadability. | Bluish tinge | A |
| 2. | Nanoemulsion formation is less than 1 min slightly less transparent, less clear | Bluish white tinge | B |
| 3. | Nanoemulsion turbid in nature formed in less than 2 min. | Milky white tinge | C |
| 4. | Nanoemulsion devoid of or minimal emulsification in 4-5 min with non uniform distribution of oil droplets. | Dull white tinge | D |
Discussion
Therapeutic agents with limited aqueous solubility like cyclosporine ezetimibe etc., show large fluctuations in peak plasma concentrations leading to decreased performance of the drug and patient noncompliance. SNEDDS can offer a reduction in ratio of bioavailability and can offer reproducibility in plasma profiles of drugs. The ability of the SNEDDS in improving Cmax and oral bioavailability or therapeutic effect has been established for various hydrophobic drugs. The improvement in bioavailability can be translated into reduction in the drug dose and dose-related side effects of many hydrophobic drugs, such as antihypertensive, antidiabetic drugs etc.
SNEDDS are used to enhance the solubility of some natural compounds like carotinoid lutein, curcumin . The bioavailability of S-SNEDDS lutein compared with the lutein powder increased to about 11.7 folds10.Taking curcumin into account, the permeability of curcumin has increased which contributed to the oral bioavailabilty of the curcumin 11. SNEDDS have also improved the absorption of anti-malarial compounds like halofantrine, cinnarizine. Halofantrine was also found to be with increased physical stability of 6 months 12. In case of cinnarizine, SNEDDS formulation showed higher dissolution rate when compared to that of the marketed tablet (Stugeron®). Higher drug release rates were observed when medium chain mixed glycerides was used and this lead to the enhanced oral bioavailability of cinnarizine. This also enhances physical and chemical stability of the drug. Hence the design of this formulation mainly relies upon the type of lipid included in it 13.
The selection of carrier is an important factor in designing solid SNEDDS as it influences factors like crystalline properties, dissolution and oral bioavailability of the drugs. Here NSAIDS such as flurbiprofen are formulated as solid SNEDDS using hydrophilic and hydrophobic carriers, in which, silicon di oxide, a hydrophobic carrier showed excellent improvement in the properties14. SNEDDS are used for enhancing the solubility of anti-inflammatory drugs such as indomethicin 15. Fibrinolytic drugs such as simvastatin, atorvastatin, valsartan, gemfibrozil were also formulated as SNEDDS for improved bioavailability. Super- SNEDDS of simvastatin shows increased bioavailability compared to the conventional SNEDDS due to the increased drug loading 16.Solid SNEDDS of valsartan enhanced the bioavailabilty potential due to the presence of porous carriers and also showed stability for about 6 months which is an important factor 17.Hormones such as ondasteron hydrochloride and insulin were also delivered orally by using SNEDDS. Solid SNEDDS of ondasteron showed increased bioavailbility than the pure drug 18. Insulin was formulated into SNEDDS by first forming Insulin-phospholipid complex(IPC) and this was used as oil phase in the formulation. This showed good hypoglycemic effect in diabetic wistar rats o oral administration. Hence IPC can be used for the oral delivery of insulin19.
Anti-cancer drugs were also formulated as SNEDDS. They include Raloxifene hydrochloride, cyclosporine A, Paclitaxel, Flutamide. In Raloxifene, the uptake of the drug by endocrine organs was assessed by administering the SNEDDS in alkalinized and non-alkalinized form to wistar rats. Non-alkalinized form showed good uptake by the endocrine organs than the alkalinized form20. SNEDDS pellets of cyclosporine A were formulated by fluid bed coating technique and this improved the in vivo performance of the drug 21. The drug release profile of Paclitaxel was improved by SNEDDS 22 and the dissolution rate was also faster compared to that of the pure drug in flutamide 23. Ezetimibe widely used in treatment of homozygous familial hypercholesterolemia and homozygous sitosterolemia is a poorly water soluble drug. This was formulated as SNEDDS for improving the bioavailability of the drug. It showed increased diffusion and absorption rate compared to that of the plain drug and marketed formulation 24. Sertraline, which is an anti-depressant was formulated as SNEDDS in order to improve the solubility. The formulation showed higher drug release and found to be stable over a period of 3 months. 25.
The potential of SNEDDS was studied using lipophilic phenothiazines, thioridazine and chlorpromazine along with the isolated plasma derived chylomicron (CM). It was observed that SNEDDS loaded with phenothiazines were uptaken efficiently by the plasma derived chylomicron26. A poorly water-soluble beta-blocker talinolol was formulated in the form of SNEDDS to enhance its bioavailability. The solubility, drug release and permeability were also found to be increased than the standard drug suspension27.
SNEDDS formulation of carvedilol (cardiovascular drug) improved the period of stability over six months and also enhanced the bioavailability. Liquid SNEDDS and also superporous hydrogel loaded with carvedilol SNEDDS were prepared which showed increase in the drug release28. Lercanidipine, which is employed as a calcium channel blocker was formulated as SNEDDS to improve its oral bioavailability. Due to the nanosized system, the formulation also enhanced the drug absorption6.
For drugs like Irbesartan, lacidipine and Adefovir dipivoxil (anti-viral drug), the dissolution rate and oral bioavailability could be effectively increased by developing the SNEDDS formulations29,30. Sildenafil citrate is the mostly used drug to treat erectile dysfunction. Nanoemulsions and SNEDDS formulation loaded with sildenafil citrate were prepared. It was observed that SNEDDS formulation showed effective oral delivery and nanoemulsions was found to be promising for transdermal permeation of the drug 31. Glibenclamide, when formulated as SNEDDS enhanced the rate of absorption due to the nanosized particles and also there was no incompatibility or interaction between the ingredients 32.
Conclusion
Ease of manufacture and scale-up is one of the most important advantages that make SNEDDS unique when compared with other novel drug delivery systems, such as solid dispersions, liposomes and nanoparticles. SNEDDS require very simple and economical manufacturing facilities, such as simple mixer with an agitator and volumetric liquid filling equipment for large-scale manufacturing. These SNEDDS are better formulation for drugs with poor solubility. This gives good absorption profiles thus offering high bioavailability for such drugs when administered orally. Hence this can be used in near future and this will solve problems related to poor solubility of drugs. In general, it is believed that nanoscale offers better transport properties and is a major driving factor for the augmented therapeutic efficacy of drugs. However, the role of nanoscale in improving the transport of drug across biological membranes and therapeutic efficacy is debatable in the case of nanoemulsions. The amenability of converting SNEDDS into solid self-nanoemulsifying systems enables development into solid dosage form. Thus, the solid self-nanoemulsifying system can serve as platform technology for delivering poorly soluble drugs. Although a lot of research is being carried out in this area, other aspects, such as in vitro/in vivo correlation, need to be establishe.
References
- Preshita P, Desai, Abhijit A, Vandana B, Patravale, Overcoming poor oral bioavailability using nanoparticle formulations – opportunities and limitations, Elsevier Ltd, 2011, 1740-6749.
- Shahba AAW, Mohsin K, Alanazi1 FK, Novel Self-Nanoemulsifying Drug Delivery Systems (SNEDDS) for Oral Delivery of Cinnarizine: Design, Optimization, and In-Vitro Assessment, AAPS PharmSciTech, 13, No. 3, 2012, DOI: 10.1208/s12249-012-9821-4.
- Jain S, Jain AK, Pohekar M, Thanki, Novel self-emulsifying formulation of quercetin for improved in vivo antioxidant potential:Implicationsfor drug-induced cardiotoxicity and nephrotoxicity, Free Radical Biology and Medicine, 65, 2013, 117–130.
- Vivek P, Chavda, Are SMEDDS and SNEDDS Same? A Gimmick Or Pharmaceutically Relevant, Mintage journal of Pharmaceutical & Medical Sciences, 2012, 7-10.
- Singh B, Singh R, Bandyopadhyay S, Kapil R, Garg B, Optimized nanoemulsifying systems with enhanced bioavailability of carvedilol, Colloids and Surfaces B: Biointerfaces, 101, 2013, 465– 474.
- Parmar N, Singla N, Amin S, Kohli K, Study of cosurfactant effect on nanoemulsifying area and development of lercanidipine loaded (SNEDDS) self nanoemulsifying drug delivery system, Colloids and Surfaces B: Biointerfaces, 86, 2011, 327–338.
- Hiral A, Makadia, Ami Y, Bhatt, Ramesh B, Parmar, Jalpa S, Paun, Tank HM, Self-nano Emulsifying Drug Delivery System (SNEDDS): Future Aspects, Asian J. Pharm. Res, 3, 2013, 21-27.
- Kang JH, Oh DH, Oh YK, Yong CS, Choi HG, Effects of solid carriers on the crystalline properties, dissolution and bioavailability of flurbiprofen in solid self-nanoemulsifying drug delivery system (solid SNEDDS), European Journal of Pharmaceutics and Biopharmaceutics, 80 (2), 2012, 289-297
- Beg S, Jena SS, Patra CN, Rizwan M, Swain S, Sruti ME, Rao B, Singh B, Development of solid self-nanoemulsifying granules (SSNEGs) of ondansetron hydrochloride with enhanced bioavailability potential, Colloids and Surfaces B: Biointerfaces, 101, 2013, 414– 423.
- Gupta S, Chavhan S, Krutika K, Sawant, Self-nanoemulsifying drug delivery system for adefovir dipivoxil: Design, characterization, in vitro and ex vivo evaluation, Colloids and Surfaces A: Physicochem. Eng. Aspects, 392, 2011, 145– 155.
- Yoo JH, Shanmugam S, Thapa P, Lee ES, Balakrishnan P, Baskaran R, Yoon SK, Choi HG, Yong CS, Yoo BK, Han K, Novel self-nanoemulsifying drug delivery system for enhanced solubility and dissolution of lutein, Archives of Pharmacal Research, 33 (3), 2010, 417-426.
- Wahlang B, Kabra D, Pawar YB, Tikoo K, Bansal AK, Contribution of formulation and excipients towards enhanced permeation of curcumin, Arzneimittel-Forschung/Drug Research, 62 (2), 2012, 88-93.
- Thomas N, Holm R, Müllertz A, Rades T, In vitro and in vivo performance of novel supersaturated self-nanoemulsifying drug delivery systems (super-SNEDDS), Journal of Controlled Release, 160 (1), 2012, 25-32.
- Larsen AT, Ohlsson AG, Polentarutti B, Barker RA, Phillips AR, Abu-Rmaileh Rd, Dickinson PA, Abrahamsson B, Østergaard J, Müllertz A, Oral bioavailability of cinnarizine in dogs: Relation to SNEDDS droplet size, drug solubility and in vitro precipitation, European Journal of Pharmaceutical Sciences, 48 (1-2), 2013, 339-350.
- Obitte NC, Ofokansi KC, Nzekwe IT, Esimone CO, Okoye IE, Self-nanoemulsifying drug delivery systems based on melon oil and its admixture with a homolipid from Bos indicus for the delivery of indomethacin, Tropical Journal of Pharmaceutical Research, 10 (3), 2011, 299-307.
- Thomas N, Holm R, Garmer M, Karlsson JJ, Müllertz A, Rades T, Supersaturated self-nanoemulsifying drug delivery systems (Super-SNEDDS) enhance the bioavailability of the poorly water-soluble drug simvastatin in dogs, AAPS Journal, 15 (1), 2013, 219-227.
- Beg S, Swain S, Singh H.P, Patra CN, Rao MB, Development, optimization, and characterization of solid self-nanoemulsifying drug delivery systems of valsartan using porous carriers, AAPS PharmSciTech, 13 (4), 2013, 1416-1427.
- Beg S, Jena SS, Patra CN, Rizwan M, Swain S, Sruti J, Rao MEB, Singh B, Development of solid self-nanoemulsifying granules (SSNEGs) of ondansetron hydrochloride with enhanced bioavailability potential, Colloids and Surfaces B: Biointerfaces, 101, 2013, 414-423.
- Zhang Q, He N, Zhang L, Zhu F, Chen Q, Qin Y, Zhang Z, Zhang Q, Wang S, The in vitro and in vivo study on Self-Nanoemulsifying Drug Delivery System (SNEDDS) based on insulin-phospholipid complex, Journal of Biomedical Nanotechnology, 8 (1), 2012, 90-97.
- Elsheikh MA, Elnaggar YSR, Gohar EY, Abdallah OY, Nanoemulsion liquid preconcentrates for raloxifene hydrochloride: Optimization and in vivo appraisal, International Journal of Nanomedicine, 7, 2012, 3787-3802.
- Lei Y, Qi J, Nie S, Hu F, Pan W, Lu Y, Wu W, Solid self-nanoemulsifying cyclosporine a pellets prepared by fluid-bed coating: Stability and bioavailability study, Journal of Biomedical Nanotechnology, 8 (3), 2012, 515-521.
- Sun M, Han J, Guo X, Li Z, Yang J, Zhang Y, Zhang D, Design, preparation and in vitro evaluation of paclitaxel-loaded self-nanoemulsifying drug delivery system, Asian Journal of Pharmaceutical Sciences, 6 (1), 2011, 18-25.
- Jeevana JB, Sreelakshmi K, Design and evaluation of self-nanoemulsifying drug delivery system of flutamide, Journal of Young Pharmacists, 3 (1), 2011, 4-8.
- Dabhi MR, Limbani MD, Sheth NR, Preparation and in vivo evaluation of self-nanoemulsifying drug delivery system (SNEDDS) containing ezetimibe, Current Nanoscience, 7 (4), 2011, 616-627.
- Rahman MA, Iqbal Z, Hussain A, Formulation optimization and in vitro characterization of sertraline loaded self-nanoemulsifying drug delivery system (SNEDDS) for oral administration, Journal of Pharmaceutical Investigation, 42 (4), 2012, 191-202.
- Shahnaz G, Hartl M, Barthelmes J, Leithner K, Sarti F, Hintzen F, Rahmat D, Salvenmoser W, Bernkop-Schnürch A, Uptake of phenothiazines by the harvested chylomicrons ex vivo model: Influence of self-nanoemulsifying formulation design, European Journal of Pharmaceutics and Biopharmaceutics, 79 (1), 2011, 171-180.
- Ghai D, Sinha VR, Nanoemulsions as self-emulsified drug delivery carriers for enhanced permeability of the poorly water-soluble selective β1-adrenoreceptor blocker Talinolol, Nanomedicine: Nanotechnology, Biology, and Medicine, 8 (5), 2012, 618-626.
- Singh B, Khurana L, Bandyopadhyay S, Kapil R, Katare OOP, Development of optimized self-nano-emulsifying drug delivery systems (SNEDDS) of carvedilol with enhanced bioavailability potential, Drug Delivery, 18 (8), 2011, 599-612.
- Patel J, Patel A, Raval M, Sheth N, Formulation and development of a self-nanoemulsifying drug delivery system of irbesartan, Journal of Advanced Pharmaceutical Technology and Research, 2 (1), 2011, 9-16.
- Basalious EB, Shawky N, Badr-Eldin SM, SNEDDS containing bioenhancers for improvement of dissolution and oral absorption of lacidipine. I: Development and optimization, International Journal of Pharmaceutics, 391 (1-2), 2010, 203-211.
- Elnaggar YSR, El-Massik MA, Abdallah OY, Self-nanoemulsifying drug delivery systems of tamoxifen citrate: Design and optimization, International Journal of Pharmaceutics, 380 (1-2), 2009, 133-141.
- Singh SK, Prasad Verma PR, Razdan B, Glibenclamide-loaded self-nanoemulsifying drug delivery system: Development and characterization, Drug Development and Industrial Pharmacy, 36 (8), 2010, 933-945.








