Ndubuisi N. Nwobodo
Department of Pharmacology and Therapeutics, Faculty of Clinical Medicinem, Ebonyi State University, Abakaliki, Nigeria.
Corresponding Author E-mail: nnwobodo@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/438
Abstract
The 3-HMG-CoA reductase inhibitors (statins) which are substrates of OATP1B1 transporter protein, is reputed to be effective in reducing morbidity and mortality associated with cardiovascular disease. The OATP1B1 transporter protein is encoded by the SLCO1 genes and almost exclusively expressed in human liver cells. It plays a crucial role in the hepatic uptake and clearance of many drugs including statins. There is a high probability of drug-drug interactions since statins are co-administered with other drugs which may inhibit the OATP1B1 transporter leading to elevated serum levels and statin-induced adverse effects. The OATP1B1 (SLCO1B1) genetic polymorphism is quite invaluable in influencing clinical decisions on the use of statins. Consequently, genotyping for selected SLCO1B1 variants is recommended to identify individuals at increased risk of developing adverse drug effects following statin therapy.
Keywords
Clinical significance; Genetic polymorphism; OATP1B1; SLCO1B1; Statins
Download this article as:| Copy the following to cite this article: Nwobodo N. N. Effects of Organic Anion Transporting Polypeptide (OATP1B1/SLCO1B1) Genetic Polymorphism on Statin Therapy. Biomed Pharmacol J 2013;6(2) |
| Copy the following to cite this URL: Nwobodo N. N. Effects of Organic Anion Transporting Polypeptide (OATP1B1/SLCO1B1) Genetic Polymorphism on Statin Therapy. Biomed Pharmacol J 2013;6(2). Available from: http://biomedpharmajournal.org/?p=2794 |
Introduction
The organic anion transporter protein 1B1 (OATP1B1) is the major transporter responsible for hepatic uptake of drugs and endogenous compounds. The 3-HMG-COA reductase inhibitors, commonly referred to as statins, are known to be effective in lowering cholesterol thereby drastically reducing mortality and morbidity associated with cardiovascular events. Statins which are generally acclaimed to have low therapeutic index are substrates of OATP1B1 transporter. There is the probability of these cholesterol lowering drugs being co-administered alongside other drugs which are inhibitors of the OATP1B1 transporter, resulting in higher serum drug concentration and leading to increased incidence of adverse drug effects; especially myopathy, rhabdomyolysis with associated risk of renal failure and hepatotoxicity due to elevated liver enzymes. A wide inter-individual variability exists in drug disposition and clinical response to 3-HMG-CoA reductase inhibitors. Attempts at explaining these differences has focused on genetic variations in hepatic influx and efflux transporters. A crucial step in the elimination of statins is access into the hepatocytes. OATP1B1 expressed on the basolaleral membrane of liver cells is one of the major influx transporters1-4. The translocation of drugs via active and passive mechanisms in and out of cells is mediated by these transporters which are integral membrane proteins1. The role of transporters in influencing drug response and pharmacokinetics has been well documented2. The 3-HMG-CoA reductase inhibitor pravastatin was one of the first recognized substrates for OATP1B1 transporter5. Currently, all statins in clinical use are known substrates of OATP1B16-10. This review paper examines the role of genetic variations in the OATP1B1 (SLCO1B1) transporter in the disposition and pharmacodynamic response of statins.
Characteristics of OATP1B1 (SLCO1B1) Genetic Polymorphism
The OATP1B1 comprises 12 putative membrane spanning domains and a large fifth extracellular loop11-13. The molecular mass is reduced following deglycosylation to 58KDa though the apparent molecular mass is given as 84KDa11. The solute carrier organic anion transporter 1 (SLCO1) consists of genes encoding OATP1B1 as well as other transporters including OATP1A2 (first cloned human OATP), OATP1B3 and OATP1C114,15. The OATP1B1 transporter protein is almost exclusively expressed in human liver cells, hence playing a significant role in hepatic uptake and clearance of albumin-bound amphipathic drugs. A number of transient and stable heterologous expression systems affect in vitro assessment of OATP1B1 function2. The study of influx and efflux transporters interplay in the transcellular transport of drugs is facilitated by employing stable expression of OATP1B1 in combination with efflux transporters7,16. A study that investigated the pharmacokinetic effects of SLCO1B1 variants revealed that subjects with the SLCO1B1*1B/*15 genotype had significantly reduced non-renal clearance compared with those persons with the *1B/*B genotype17. The effects of SLCO1B1c.521T>C SNP, in a series of genotype-panel studies, on the pharmacokinetics of various statins were investigated and the largest observed effect attributed to simvastatin18,19. It could be predicted, based on the concentration-dependent skeletal muscle toxicity of statins, that the low activity SLCO1BI variants may be associated with an increased risk of statin-induced myopathy18. A study reported AUC of pravastatin 35% lower in healthy individuals with SLCO1B1*1B/*1B genotype as compared to persons with the *1A/*1A genotype, consistent with an enhanced hepatic uptake in association with the *1B haplotype20. The pharmacokinetics of another 3-HMG-CoA redutase inhibitor rosuvastatin, however, seem not to be affected by the SLCO1B1/*1B haplotype21. OATP1B1 transporter is inhibited in vitro by a number of substrates, some of which are non-specific for OATP1B1 but may competitively inhibit other substrates that bind at the same site of OATP1B1. The wide variations in IC50 values of individual inhibitors on different substrates lend credence to the idea that OATP1B1 may possess multiple substrate binding sites.
Clinical Significance
The clinical implication of OATP1B1 (SLCO1B1) genetic polymorphism can be well illustrated by adverse drug effects associated with statin therapy. Statin-induced myopathy is a rare plasma concentration dependent adverse reaction22-24. This usually manifests as weakness or muscle pain associated with increased creatine kinase levels and may result to rhabdomyolysis (muscle breakdown and mygolobin release) with increased risk of renal failure and mortality. A genome wide association study involving 85 patients who developed myopathy on a high dose 80mg daily simvastatin treatment and 90 matched control subjects as part of SEARCH (Study the Effectiveness of Additional Reductions in Cholesterol and Homocysteine) trial provided a crucial evidence that the risk of simvastatin-induced myopathy is altered by at least one common variation the SLCO1B1 gene25.
The SLCO1B1c.521T>C SNP (short nucleotide polmorphism) increases risk of adverse effect during statin treatment, since statin-induced myopathy is concentration-dependent. The above study also revealed that simvastatin induced myopathy was associated with a non-coding SNP in the SLCO1B1 gene, which is in strong linkage disequilibrium with the c.521T>C SNP. Relatively milder forms of statin-induced myopathy had been associated with the SLCO1B1c.521T>C SNP despite the low dose statins 26.
Certain in vivo drug-drug interactions may be partly attributed to inhibitors of OATP1B1. The plasma concentration of statins can be markedly elevated by cyclosporine27-31. Notwithstanding, that inhibition of CYP3A4 may partly account for the effects of cyclosporine on simvastatin, lovastatin, cerivastatin, atorvastatin; but is not so for other statins such as pitavastatin, pravastatin and rosuvastatin22.
The inhibition of OATP1B1 mediated uptake of cerivastatin by the liver, resulted to a 3 to 8 fold increase in the AUC of cervastatin in kidney transplant recipients on cyclosporine immunsuppressive therapy32,33. However, another calcineurium inhibitor, tacrolimus does not affect plasma concentration of simvastatin or atorvastatin which suggests that it does not inhibit OATP1B134,35. The rate delivery elimination process should be given consideration in understanding the role of OATP1B1 transporter in hepatic drug clearance. The most important determinant of hepatic clearance is the hepatic uptake rather than the metabolic intrinsic clearance and this is influenced by genetic polymorphism of the OATP1B1 transporter36 . The pharmacokinetics of statins is influenced by genetic polymorphism of the OATP1B1 transporter, as crucial molecular determinant37. Genetic variations in OATP1B1 drug transporter influence drug response and efficacy38.
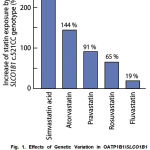 |
Fig. 1. Effects of Genetic Variation in OATP1B1/SLCO1B1 Transporter (SLCO1B1c.521T>C Variant) on Pharmacokinetic Disposition of Various Statins. |
Adapted from: Niemi M., Pasanen M.K., Neuvonen P.J. SLCO1B1 polymorphism and sex affect the pharmacokinetics of pravastatin not fluvastatin. Clin. Pharmacol.
Ther. 2006; 80:356-366 and Pasanen M.K., Friedrikson H., Neuvonen P.J., Niemi M. Different effects of SLCO1B1 polymorphism on the pharmacokinetics of atorvastatin and rosuvastatin. Clin. Pharmacol. Ther. 2007; 82: 726-733.
Conclusion
In conclusion, the OATP1B1 (SLCO1B1) genetic polymorphism has significant implication in influencing clinical decision pertaining to statin therapy. It is not only invaluable in understanding drug-drug interactions; but also influences dose adjustment with a view to minimizing adverse drug effects and enhancing clinical efficacy with statin use. Hence, gene testing (genotype) particularly for selected variants of SLCO1B1c.521T >C is recommended to determine individuals a risk of developing adverse drug effects.
References
- Klaassen C.D. and Aleksunes L.M. Xenobiotic, bile acid and cholesterol transporters: function and regulation. Pharmaol Rev. 2010; 62(1): 1-96.
- Giacomini K.M., Huang S.M., Tweedie D.J., Benet L.Z., Brouwer K.L., Chu X., Dahlin A., Evers R., Fischer V., Hillgren K.M., Hoffmaster K.A., Ishikawa T., Keppler D., Kim R.B., Lee C.A., Niemi M., Polli J.W., Sugiyama Y., Swaan P.W., Ware J.A., Wright S.H., Yee S.W., Zamek=Gliszczynski M.J. and Zhang L. Membrane transporters in drug development. Nat. Rev. Drug Discov. 2010; 9: 215- 236.
- Kalliokoski A. and Niemi M. Impact of OATP transporters on pharmacokinetics. Br J. Pharmacol. 2009; 158: 693-703.
- Fahrmayr C., Fromm M.F. and Konig J. Hepatic OATP and OCT uptake transporters: their role for drug-drug interactions and pharmacogenetic aspects. Drug Metab. Rev. 2010; 42: 380-402.
- Hsiang B., Zhu Y., Wang Z., Wu Y., Sasseville V., Yang W.P. and Kirchgessner T.G. A novel human hepatic organic anion transporting polypeptide (OATP2). Identification of liver-specific human organic anion transporting polypeptide and identification of rat and human hydroxymethyl glutaryl-CoA reductase inhibitor transporters. J. Biol. Chem. 1999; 274(52): 37161-37168.
- Furihata T., Satoh N., Ohishi T, Ugajin M., Kameyama Y., Morimoto K, Matsumoto S., Yamashita K., Kobayashi K. and Chiba K. Functional analysis of a mutation in the SLCO1B1 gene (c.1628 >G) identified in a Japanese patient with pravastatin-induced myopathy. Pharmacogenomis J. 2009; 9(3):185-193.
- Kopplow K., Letschert K., Konig J., Walter B. and Keppler D. Human hepatobiliary transport of organic anions analyzed by anodruple transfected cells. Mol. Pharmacol. 2005; 68: 1031-1038.
- kitamura S., Maeda K., Wang Y. and Sugiyama Y. Involvement of multiple transporters in the hepatobiliary transport of rosuvastatin. Drug Metab. Dipos. 2008; 36: 2014-2023.
- Noe J., Portmann R., Brun M.E. and Funk C. Substrate dependent drug-drug interactions between gemfibrozil, fluvastatin and other organic anion transporting (OATP) substrates on OATP1B1, OATP2B1, OATP1B3. Drug Metab. Dispos. 2007; 35: 1308-1314.
- Niemi M. Transporter pharmacogenetics and statin toxicity. Clin. Pharmacol. Ther. 2010; 87: 130-133.
- Konig J., Cui Y., Nies A.T. and Keppler D. A novel human organic anion transporting polypeptide localized to the basolateral hepatocyte membrane. Am. J. Physiol. Gastrointest. Liver Physiol. 2000; 278: G156- G164.
- Chang C., Pang K.S., Swaan P.W. and Ekins S. Comparative pharmacophere modeling of organic anion transporting polypeptide: a meta-analysis of rat OATP1A1 and human OATP1B1. J. Pharmacol. Exp. Ther. 2005; 314: 533-541.
- Hagenbuchi B. and Meier P.J. The super family of organic anion transporting polypeptide. Biochem. Biophys.Acta. 2003; 1609: 1-18.
- Kullak-Ublick G.A., Beuers U., Meier P.J., Domdey H. and Paumgartner G. Assignment of the human organic anion transporting polypeptide (OATP) gene to chromosome 12P12 by fluorescence in situ hybridization. J. Hepatol. 1996; 25(6): 985-987.
- Kullak-Ublick G.A., Hagenbuch B., Stieger B., Schteingart C.D., Hofmann A.F., Wolkoff A.W. and Meier P.J. Molecular and functional characterization of an organic anion transporting polypeptide cloned from human Liver. Gastroenterology. 1995; 109: 1274- 1282.
- Matsushima S., Maeda K., Kondo C., Hirano M., Sasaki M., Suzuki H. and Sugiyama Y. Identification of the hepatic efflux transporters of organic anions using double-transfected Madin-Darby canine kidney II cells expressing human organic anion transporting polypeptide 1B1 (OATP1B1) multidrug resistance 1 and OATP1B1 breast cancer resistance protein. J. Pharmacol. Exp. Ther. 2005; 314: 1059- 1067.
- Nishizato Y., Ieiri I., Suzuki H., Kimura M., Kawabata K., Hirota T., Takane H., Irie S., Kusuhara H., Urasaki Y., Urae A., Higuchi S., Otsubo K. and Sugiyama Y. Polymorphisms of OATP-C (SLC21A6) and OAT3 (SLC22A8) genes. Consequences for pravastatin pharmacokinetics. Clin. Pharmacol. Ther. 2003; 73(6): 554- 565.
- Niemi M., Pasanen M.K. and Neuvonen P.J. SLCO1B1 polymorphism and sex affect the pharmacokinetic of pravastatin but not fluvastatin. Clin Pharmacol. Ther. 2006; 80: 356-366.
- Pasanen M.K., Fredrikson H., Neuronen P.J. and Niemi M. Different effects of SLCO1B1 polymorphism on the pharmacokinetics of atorvastatin and Clin. Pharmacol. Ther. 2007; 82: 726-733.
- Maeda K, Ieiri I., Yasuda K., Fujimo A., Fujiwara H., Otsubo K., Hirano M., Kusuhara H. and Sugiyama Y. Effects of organic anion transporting polypeptide 1B1 haplotype on pharmacokinetics of pravastatin, valsartan and tenocapril. Clin. Pharmacokinet. Ther. 2006; 79(5): 427-439.
- Choi J.H., Lee M.G., Cho J.Y., Lee J.E., kin K.H. and Park K. Influence of OATP1B1 genotype on the pharmacokinetics of rosuvastatin in Koreans. Clin. Pharmacol. Ther. 2008; 83(2): 251-257.
- Neuvonen P.J., Niemi M. and Backman J.T. Drug interactions with lipid lowering drugs: mechanisms and clinical relevance. Clin. Pharmacol. Ther. 2006; 80: 565-581.
- Ghatak A., Faheem O. and Thompson P.D. The genetics of statin- induced myopathy. Atherosclerosis. 2010; 210: 337-343.
- Thompson P.D., Clarkson P. and Karas R.H. Statin associated myopathy. JAMA. 2003; 289: 1681-1690.
- SEARCH Collaborative Group, Link E., Parish S., Armitage J., Bowman L., Heath S., Matsuda F., Gut I., Lathrop M. and Collins R. SLCO1B1 variants and statin-induced myopathy: a genomewide study. N. Engl. J. Med. 2008; 359: 789-799.
- Voora D., Shah S.H., Spasojevic I., Ali S., Reed C.R., Salisbury B.A. and Ginsburg G.S. The SLCO1B1*5 genetic variant is associated with statin induced side effects. J. Am. Coll. Cardiol. 2009; 54: 1609- 1616.
- Asberg A., Hartmann A., Fieldsa E., Bergan S. and Holdaas H. Bilateral pharmacokinetic interaction between cyclosporine A and atorvastatin in renal transplant recipients. Am. J. Transplant. 1(4): 382-386.
- Arnadottir M., Eriksson L.O., Thysell H., Karkes J.D. Plasma concentration profiles of simvastatin 3-hydroxyl-3-methyl glutaryl Coenzyme A reductase inhibitory activity in kidney transplantation recipients with and without cyclosporine. Nephron. 1993; 65: 410- 413.
- Hedmen M., Neuvonen P.J., Neuvonen M., Holmberg C. and Antikainen M. Pharmacokinetic and phamacodynamics of pravastatin in pediatric and adolescent cardiac transplant recipients on a regimen of triple immunosuppression. Clin. Pharmacol. Ther. 2004; 75: 101-109.
- Park J.W., Siekmeier R., Lattke P., Merz M., Mix C., Schuler S. and Jaross W. Pharmacokinetics and pharmacodynamics of fluvastatin in heart transplant recipients taking cyclosporine. Am. J. Cardiovasc. Phamacol. Ther. 2001; 6: 351-361.
- Hasunuma T., Nakamura M., Yachi T., Arisawa N., Fukushima K., Iijima H. and Saito Y. The drug-drug interactions of pitavastatin (NK-104) a novel HMG-CoA reductase inhibitor and cyclosporine. Clin. Ther. Med. 2003; 19: 381-389.
- Muck W., Mai I., Fritsche L., Ochmann K., Johne A., Bauer S., Budde K., Roots I., Neumayer H.H. and Kuhlmann J. Increase in cerivastatin systemic exposure after single and multiple dosing, in cyclosporine-treated kidney transplant recipients. Clin. Pharmacol Ther. 1996; 65: 251-261.
- Shitara Y. and Sugiyama Y. Pharmacokinetic and pharmacodynamic alterations of 3-hydroxyl-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors: drug-drug interactions and inter-individual differences in transporter and metabolic enzyme functions. Pharmacol. Ther. 2006; 112(1): 71-105.
- Ichimaru N., Takahara S., Kokado Y., Wang J.D., Hatori M., Kameoka H., Inoue T., and Okuyama A. Changes in lipid metabolism and effect of simvastatin in renal transplant recipients induced by cyclosporine or tacroliums. Atherosclerosis. 2001; 158: 417-423.
- Lemahieu W.P., Hermann M., Asberg A., Verbeke K., Holdaas H., Vanrenterghem Y. and Maes B.D. Combined therapy with atorvastatin and calcineurium inhibitors; no interactions with tacrolimus. Am. J. Transplant. 2005; 5: 2236-2243.
- Shitara Y., Maeda K., Ikejiri K., Yoshida K., Horie T. and Sugiyama Y. Clinical significance of organic anion transporting polypeptides (OATPs) in drug disposition: their role in hepatic clearance and intestinal absorption. Biopharm. Drug Dispos. 2013; 34(1): 45-78.
- Nakanishi T. and Tamai I. Genetic polymorphism of OATP transporters and their impact on intestinal absorption and hepatic disposition of drugs. Drug Metab. Pharmacokinet. 2012; 27(1): 106-121.
- Gong I.Y. and Kim R.B. Impact of genetic variation in OATP transporters to drug disposition and response. Drug Metab. Pharmacokinet. 2013; 28(1): 4-18.







