Manuscript accepted on :
Published online on: 18-12-2015
Plagiarism Check: Yes
Tu HK Nguyen and Minh D Nguyen
School of Biotechnology, Hochiminh city International University, Ho Chi Minh City, Vietnam.
DOI : https://dx.doi.org/10.13005/bpj/409
Abstract
Phyllanthusurinariaplant was used in liver relating diseases in Vietnam. The study was to know more about the bioactivities of Phyllanthusurinarialeaf extracts before and after combined with Pandanustectoriousfruit extracts and Lactobacillus rhamnosusextract. This was accomplished by examining the influence of the total aqueous, chloroform and ethylacetateextracts. The biological tests were done onCandida albicans,Salmonella typhi, Pseudomonas aeruginosaand Staphylococcus aureus.Agar diffusion test was applied to determine the antimicrobial activities.Based on the inhibition zone diameter, the result showed that Phyllanthusurinariaaqueousextraction had the inhibition on the Pseudomonas aeruginosa(21.75 ± 0.96 mm)and Staphylococcus aureus(24.75 ± 0.96 mm). The activities decreased after combined with Pandanustectoriousfruit extractson Pseudomonas aeruginosa(14.75 ± 0.50 mm) and Staphylococcus aureus(14.75 ± 0.50 mm)and Lactobacillus rhamnosus(14.75 ± 0.50 mm).The total aqueous extracts fractionated with chloroform and ethylacetate still showed activities that suggested the polar and nonpolar antimicrobial compounds existed in Phyllanthusurinaria. However, the activities of these fractionated extracts in the combination showed the weaker activities than in the using only Phyllanthusurinariaextracts. Especially, there was no activity of the ethylacetate extracts in the combination with Lactobacillus rhamnosus. The study is the first report of the combination of Phyllanthusurinaria with Pandanustectorious and Lactobacillus rhamnosus PN04.
Keywords
Phyllanthusurinaria; Pandanustectorius; Lactobacillus rhamnosus; Antimicrobial activity
Download this article as:| Copy the following to cite this article: Nguyen T. H, Nguyen M. D. Antimicrobial Activities of Phyllanthus urinaria Extracts Before and After Combined with Pandanus tectorious and Lactobacillus rhamnosus PN04. Biomed Pharmacol J 2013;6(2) |
| Copy the following to cite this URL: Nguyen T. H, Nguyen M. D. Antimicrobial Activities of Phyllanthus urinaria Extracts Before and After Combined with Pandanus tectorious and Lactobacillus rhamnosus PN04. Biomed Pharmacol J 2013;6(2). Available from: http://biomedpharmajournal.org/?p=2704 |
Introduction
Traditional medicine has been used in some communities for thousands of years without understanding on safety, effectiveness and quality. Many kinds of traditional medicines caused the side effects (Niggeman and Gruber, 2003). Some of them are safety, effectiveness and quality. Therefore, finding the bioactivities of traditional medicine was necessary.Phyllanthusurinaria (P. urinaria), one of the herbal plants belonging to the genus Phyllanthus (Euphorbiaceae), is widely distributed in China,South India and Southern America. The ethylacetate extract of P. urinaria was shown to exhibit anticancer activity by inducing apoptosis through the inhibition of telomerase activity and Bcl-2 expression (Huang et al., 2003; Huang et al., 2004a and 2004b).The water extract prepared from P. urinaria has an anticancer effect on Lewis lung carcinoma cells through a similar pathway (Huang et al., 2006).One of the most commonly used traditional herbs in Vietnam is Phyllanthusurinaria. People usually used this herb to treat many diseases as hepatitis, diuretic, jaundice, edema, pimples. Sometimes, people combined Phyllanthusurinariawith some other medical herbs to treat various kinds of diseases as Pandanustectorious(also called pineapple wood, family of Pandanaceae) which was used to treat cough, hemorrhoids, dysentery.Pandanustectorius (P. tectorius) is a large shrub or small tree of immense cultural, health, and economic importance in the Pacific where it can withstand drought, strong winds, and salt spray. The nutritious fruits are edible varieties, those with low amounts of calcium oxalate crystals. One 100 g portion of edible pericarp is mainly comprised of water (80 g) and carbohydrates (17 g). There are also significant levels of betacarotene (19 μg to 19 mg) and vitamin C (5 mg), and small amounts of protein (1.3 mg), fat (0.7 mg), and fiber (3.5 g) (Englberger et al. 2003, Englberger et al. 2006a and 2006b).Besides that, in order to improvement the dysentery, constipation and hepatitis, people used probiotic popularly in their life. Probiotics are living microorganisms, which, when ingested or locally applied in sufficient numbers, provide the consumer with one or more proven health benefits(De Keersmaecker et al., 2006; Chateris, 1998).One of probiotic used in colon treatment was Lactobacillus rhamnosus(Avlami, 2001).Lactobacillus rhamnosus is usually isolated from milk, human vagina. Therefore, in order to study the combination of Phyllanthusurinariawith Lactobacillus rhamnosus, Lactobacillus rhamnosus PN04 isolated in plant named HottuyniacordataThunb was chosen for the study (Nguyen et al., 2013).
Additionally, to confirm that if people combinedPhyllanthusurinaria with Pandanustectorious(fruit extracts), the antimicrobial activities of Phyllanthusurinaria extracts with or without combination with Pandanustectorious(fruit extracts) was done.
Materials and Methods
Bacterial strains and growth conditions
Five pathogen strains wereSalmonella typhi, Candida albicans, Pseudomonas aeruginosaand Staphylococcus aureus. These bacteria have been cultured and maintained in LB (Luria-Bertani) broth and agar.
Lactobacillus rhamnosus PN04isolated from Houttuyniacordata.Thunb(Nguyen et al., 2013) was used in this study. This strain was maintained in MRS agar and MRS broth. The MRS medium contains peptone, glucose, yeast extract, tween 80, dipotassium phosphate, sodium acetate, ammonium citrate, magnesium sulphate and manganese sulphate(De Man et al., 1960).
Herbs
Dry Phyllanthusurinaria and Pandanustectoriuswere bought commercially from medical store located in HaiThuongLanOngstreet, district 5, Ho Chi Minh City, Vietnam.
Total water extraction
Fifty grams of dry Phyllanthusurinariaand fifty grams of Pandanustectoriuswere boiled with water enough (50 mL) for 1 hour to obtain a volume of 20 mL.The extracts were collected, thenanother water portion (50 mL) was added into this mixture and boiled continuously for 1 hour to obtain 20 mL. The second extract was collected and combined with the former for antimicrobial tests.
Fractionated extraction with chloroform
The water extraction was fractionated with chloroform. 10 mL of total aqueous extract was mixed with 3 mL of chloroform. The mixture of total was shaken well for 30 minutes and the lower layer was collected.This procedure was performed in triplicate. The chloroform extracts were combined for antimicrobial tests.
Fractionated extraction with ethylacetate
After completely extracted with chloroform, this extract was shakenwith 3 mL of ethylacetate for 30 minutes. The mixture was separated in three layers. The lowest layer was collected and the extraction with ethylacetatewas done for more twice. All the lowest extracts were combined and ready for antimicrobial activity test.
Antimicrobial activity tests
Antimicrobial effects were tested on the pathogens by the agar diffusion method. The tested microorganismswere propagated twice and then grown for 18-24 h in 10 ml of appropriate growth media. Turbidity of the culture broth was compared with McFarland tubes to give an estimate of bacterial population (106 CFU/mL). Supernatant of the cell after expression were collected after centrifugation at 12,000 rpm for 15 min) and the clear supernatantwas sterilized by filtration (0.45 μm), thus yielding cell-free filtrates. The wells (ø 6 mm) were then prepared and filled using 100 μL of cell-free filtrate. The inoculated plates were incubated for 18-24 h at appropriate temperatures, and the diameter of the inhibition zone was measured in millimeters with calipers. The measurements recorded werefrom the edge of the zone to the edge of the wall.
Results and Discustion
Antimicrobial activities of the water extracts
The objective of this project was to find out the antimicrobial activities of P.urinaria and P. urinaria combined with P.tectoriuswhen extract with water. The result showed that the different amount applied into wells gave significant difference (p<0.05). From Table 1, the inhibition of the total extract of P.urinariaon S.aureus(24.75 ± 0.96 mm)was significantly stronger than P.aeruginosa(21.75 ± 0.96 mm)based on the inhibition zone diameter.The total extract of the combination between P.urinaria and P.tectoriusshowed the weaker activities than the total extract of the P.urinariaonS. aureus(14.75 ± 0.50 mm)was bigger than P. aeruginosa(14.75 ± 0.50 mm). There was no activity on Candida albicans and Salmonella typhi. The antimicrobial activities of the aqueous extract were not broad. Regarding to the inhibition on S. aureus, P.urinariamight be used in skin infection and sepsis. According to Richard RM (1973), psoriatic patients were studied with regard to the quantities of S.aureus on involved and uninvolved skin. About 50% of the patients carried S.aureus, usually in low numbers. All patients with erythroderma harbored S.aureus, mostly on their skin. In atopic dermatitis, sepsis and skin infections, toxin C and in psoriasis, toxin B was most often detected. S.aureus was present in more than 50% of patients with atopic dermatitis and psoriasis. The severity of AD and PS significantly correlated to enterotoxin production of the isolated S aureus strains(Nordwi, 2005). As a result, Phyllanthusurinaria can aid for the skin diseases as psoriasis and atopic dermatistis.
Table 1: Antimicrobial activity test of the water extract ofPhyllanthusurinariaaccording to the inhibition zone diameter (mm)
| Pathogens | Inhibition zone diameter (mm) | |
| Phyllanthus | Phyllanthus and Pandanus | |
| Pseudomonas aeruginosa | 21.75 ± 0.96 | 14.75 ± 0.50 |
| Staphylococus aureus | 24.75 ± 0.96 | 14.75 ± 0.50 |
| Candida albicans | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Salmonella typhi | 0.00 ± 0.00 | 0.00 ± 0.00 |
Mean±SD
P.aeruginosa is an important bacterial pathogen, particularly as a cause of infections in hospitalised patients, immunocompromised hosts and patients with cystic fibrosis. Surveillance of nosocomial P. aeruginosa infections has revealed trends of increasing antimicrobial resistance, including carbapenem resistance and multidrug resistance. Mechanisms of antimicrobial resistance include multidrug efflux pumps, ß-lactamases and downregulation of outer membrane porins. Mechanisms of virulence include secreted toxins and the ability to form biofilms. The effective treatment of infections caused by P. aeruginosa includes prevention when possible, source control measures as necessary and prompt administration of appropriate antibacterial agents. Antibacterial de-escalation should be pursued in patients with an appropriate clinical response, especially when antibacterial susceptibilities are known (Driscoll, 2007). Multidrug-resistant P. aeruginosa may require treatment with less commonly used antibacterials (e.g. colistin), but newer anti-pseudomonalantibacterials are expected to be available in the near future.P. urinaria may be used in bacteremia, diarrhea, ecthymagangrenosum in treating multidrug-resistant P. aeruginosa.
AlthoughPhyllanthus had an effect on DNA polymerase of virus (Joseph, 2011), the mechanism of this plant on the examined bacteria should be studied more. However, there were the significant differences (p<0.05) between total extract solutions of P.urinaria before and after combined with P.tectorius onP.aeruginosa, S.aureus. The total extract of P.urinariashowed the stronger than the combination with P.tectorius.The study informed that the combination of these two herbs in treating diseases related to these kinds of bacteria should be considered.
Antimicrobial activity of the extractsfractionated with chloroform and ethylacetate
The Table 2 and Table 3 showed the activities of the extracts fractionated with chloroform or in the extract after ethylacetate. In P.urinaria, there are some antimicrobial compounds in both polar and nonpolar. Interestingly, the activities decreased after these chloroform and ethylacetateextracts of P.urinariain the combination with P.tectorius.From Table 2, the inhibition of the chloroform extract of P.urinariaon S.aureus(18.25 ± 0.50mm) was significantly stronger than P.aeruginosa (17.25 ± 0.50mm) based on the inhibition zone diameter.The combination of the chloroform extract of P.urinaria and P.tectorius showed the weaker activities onS.aureus(14.25 ± 0.50mm)andP.aeruginosa(10.25 ± 0.50 mm).However, The combination of the chloroform extract of P.urinaria and P.tectorius showed the similar activity onS.aureus(14.75 ±0.50 mm). The antimicrobial characteristics of P.urinaria and P.tectorius might have interestingmehanisms. The compounds in these plants might be antagonists. The identification of P.urinaria compounds will be done so far.
Table 2: Antimicrobial activity test of the chloroform extract ofPhyllanthusurinariaaccording to the inhibition zone diameter (mm)
| Pathogens | Inhibition zone diameter (mm) | |
| Phyllanthus | Phyllanthus and Pandanus | |
| Pseudomonas aeruginosa | 21.75 ± 0.96 | 10.25 ± 0.50 |
| Staphylococus aureus | 18.25 ± 0.50 | 14.25 ± 0.50 |
Mean±SD
From Table 3, the inhibition of the ether extract of P. urinaria on S.aureus (17.75 ± 0.50mm) was significantly stronger than P.aeruginosa (16.25 ± 0.50mm) based on the inhibition zone diameter.The combination of the ether extract of P.urinaria and P.tectorius showed the weaker activities on S.aureus (11.50 ± 0.58 mm)and P.aeruginosa (9.5 ± 0.58 mm). The inhibition of the ether extract of P.urinaria on S.aureusand P. aeruginosa was insignificantly different from the inhibition of the chloroform extract of P. urinaria stronger than inP.aeruginosa (p<0.5).
Table 3: Antimicrobial activity test of the ethylacetate extract of Phylanthus urinariaaccording to the inhibition zone diameter (mm)
| Pathogens | Inhibition zone diameter (mm) | |
| Phyllanthus | Phyllanthus and Pandanus | |
| Pseudomonas aeruginosa | 16.25 ± 0.50 | 17.75 ± 0.50 |
| Staphylococus aureus | 9.5 ± 0.58 | 11.5 ± 0.58 |
Mean±SD
Antimicrobial activity of Lactobacillus rhamnosusPN04
In order to study the effects of Lactobacillus rhamnosus PN04 on the extracts of P.urinaria, the antimicrobial activity of Lactobacillus rhamnosus was tested. As showing in Table 4 and Figure 1,L.rhamnosusPN04 isolated in HottuyniacordataThunb had the highest inhibition on Salmonella typhi(11.00±1.00), Pseudomonas aeruginosa (14.25 ± 0.50), Staphylococcus aureus (18.5 ± 0.58) in exponential phase. Regarding to the inhibition of Phyllanthusurinaria on Staphylococcus aureus and Pseudomonas aeruginosa, the supernatant of Lactobacillus rhamnosus PN04 was used in the combination of Phyllanthusurinaria on S. aureus and P. aeruginosa.
Table 4: Antimicrobial activity test of Lactobacillus rhamnosus according to the inhibition zone diameter (mm)
| Pathogens | Inhibition zone diameter (mm) | |||
| Early exponential phase | Late exponential phase | Stationary phase | Death phase | |
| Salmonella typhi | 0.00 ± 0.00 | 0.00 ± 0.00 | 11.00±1.00 | 10.00± 1.00 |
| Pseudomonas aeruginosa | 0.00 ± 0.00 | 7.67± 2.03 | 14.25 ± 0.50 | 11.67 2.31 |
| Staphylococus aureus | 0.00 ± 0.00 | 5.33 ± 0.58 | 18.5 ± 0.58 | 8.33 ± 2.08a |
| Candida albicans | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
Mean±SD
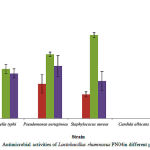 |
Figure 1: Antimicrobial activities of Lactobacillus rhamnosus PN04in different phases |
Antimicrobial activity of Lactobacillus rhamnosusandPhyllanthusurinaria and Pandanustectorus
As shown in Table 5 and Figure 2, L. rhamnosus reduces the activity of P. urinaria on Pseudomonas aeruginosa. Especially, there was resistance on P. aeruginosa and S. aureus when the ethyacetate fraction combined with Pandanustectorius and L. rhamnosus. However, the ethyl acetate fraction and L.rhamnosus showed the insignificant difference of the activities on two pathogens. In this case, the activities were not significant different from the extracts without combination. Similarly, the chloroform fractions did not show the significance on the activities of the combined extracts. To understand the combination, more studies are carried out. The combination in using these plants and L. rhamnosus was complicated. People should not usedL. rhamnosus and P.urinaria together in treating some diseases that related to intestinal like dysentery, constipation.
Table 5: Antimicrobial activity test of the aqueous, chloroform, ethylacetate extract of Phyllanthusin the combination ofPandanus and Lactobacillusaccording to the inhibition zone diameter (mm)
| Pathogens | Inhibition zone diameter (mm) | ||||||
| L. rhamnosus | Phylanthus and L. rhamnosus | Phylanthus and Pandanus and L. rhamnosus | Choroform extract of Phylanthus and L. rhamnosus | Choroform extract of Phylanthus and Pandanus and L. rhamnosus | Ethyl acetate extract of Phylanthus and L. rhamnosus | Ethylacetate extract of Phylanthus and Pandanus and L. rhamnosus | |
| Pseudomonas aeruginosa | 14.25 ± 0.50 | 4.75 ± 5.50 | 4.75 ± 5.50 | 16.25 ± 0.50 | 13 ± 0.82 | 12.25 ± 0.96 | 00 ± 0.00 |
| Staphylococcus aureus | 18.5 ± 0.58 | 14.25 ± 0.96 | 14.25 ± 0.96 | 15 ± 0.82 | 13.25 ± 1.50 | 15.25 ± 0.50 | 00 ± 0.00 |
Mean±SD
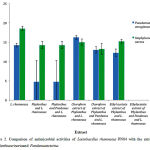 |
Figure 2: Comparison of antimicrobial activities of Lactobacillus rhamnosus PN04 with the extracts of Phyllanthusurinariaand Pandanustectorius
|
In Viet Nam, the traditional method that people used to extract this kind of herbal is water extraction, so that some characteristics might loss or might not be secreted during the extraction. This lead to the result that samples only inhibited the activities of two indicators bacteria as P. aeruginosa and S. aureus. According to the previous study, P.amarus, a plant related with Phyllanthusurinaria when extract with ethanol may inhibit the activity of Salmonella typhi(Oluwafemi and Debiri, 2008). Moreover, the Phyllanthusurinaria can also inhibit Pseudomonas aeruginosa, Staphylococcusaureusand another microorganismas Escherichia coli, Bacillus cereus,Klebsiellaaerogenes, Proteus vulgaris, Shigellaboydis when extract of Phylanthusurinaria with acetone or methanol (Daburet. al., 2007). Our study has found the antimicrobial activities on S. aureus and P. aeruginosa of the water extract of P. urinaria. Also, the study warned the people who used these plants and L. rhamnosus for any treatment should be carefully.
Conclusion
The study showed the antagonistic characteristics of Phyllanthusurinaria and Pandanustectorius and Lactobacillus rhamnosus on Staphylococcus aureus and Pseudomonas aeruginosa. With the action on Staphylococcus aureus and Pseudomonas aeruginosa, Phyllanthusurinaria could be used in treating psoriasis, atopic dermatitis, bacteremia, diarrhea, ecthymagangrenosum. However, the mechanism of action should be done.
References
- Avlami, A. Lactobacillus rhamnosus endocarditis complicating colonoscopy. , 2001; 42(4): 283–285.
- Charteris, W. Ingredient selection criteria for probiotic micro-organisms in functional dairy foods. J. Dairy Technol., 1998; 51: 123-136.
- Dabur, R. Antimicrobial activity of some indian medicinal plants. J. Tradit. Complement. Altern. Med.,2007; 4 (3): 313 – 318.
- De Keersmaecker, S.C., Verhoeven, T.L., Desair, J., Marchal, K., Vanderleyden, J., Nagy, I.Strong antimicrobial activity of Lactobacillus rhamnosus GG against Salmonella typhimurium is due to accumulation of lactic acid. FEMS Microbiol. Lett., 2006; 259(1): 89-96.
- De Man, J.D., Rogosa, M., Sharpe, M.E., A medium for the cultivation of Lactobacilli. Appl. Bact.1960; 23: 130-135.
- Driscoll, J.A., Brody, S.L., Kollef, M.H. The epidemiology, pathogenesis and treatment of Pseudomonas aeruginosa Drugs., 2007; 67(3):351-68.
- Englberger, L., Aalbersberg, W., Dolodolotawake, U., Schierle, J., Humphries, J., Iuta, T., Marks, G.C., Fitzgerald, M.H., Rimon, B., Kaiririete, M. Carotenoid content of pandanus fruit cultivars and other foods of the Republic of Kiribati. Public Health Nutr., 2006a; 9(5): 631-643.
- Englberger, L., Aalbersberg, W., Fitzgerald, M.H., Marks, G.C., Chand, K. Provitamin A carotenoid content of different cultivars of edible pandanus fruit. Food. Comp. Anal., 2003a; 16: 237–247.
- Englberger, L., Fitzgerald, M.H., Marks, G.C. Pacific pandanus fruit: an ethnographic approach to understanding an overlooked source of provitaminA carotenoids. Asia. Pac J ClinNutr, 2003b; 12: 38–44.
- Englberger, L., W. Aalbersberg, J., Schierle, G.C., Marks, M.H., Fitzgerald, F., Muller, A., Jekkein, J., Alfred, N., van der Velde. Carotenoid content of different edible pandanus fruit cultivars of the republic of the Marshall Islands. Food Comp. Anal., 2006 b; 19: 484 – 494.
- Huang, S.T, Yang, R.C, Yang, L.J., Lee, P.N., Pang, J.H.S. Phyllanthusurinaria triggers the apoptosis and Bcl-2 down-regulation in Lewis lung carcinoma cells. Sci., 2003; 72:1705-1716.
- Huang, S.T., Yang, R.C., Chen, M.Y., Pang, J.H.S. Phyllanthusurinaria induces the Fas receptor/ligand expression and ceramide-mediated apoptosis in HL-60 Cells. Sci., 2004; 75(3): 339-351.
- Huang, S.T., Yang, R.C., Lee, P.N., Yang, S.H., Liao, S.K., Chen, T.Y., Pang, J.H.S. Anti-tumor and anti-angiogenic effects of Phyllanthusurinaria in mice bearing Lewis lung carcinoma. Immunopharmacol.,2006; 6: 870-879.
- Huang, S.T., Yang, R.C., Pang, J.H.S. Aqueous extract of the Phyllanthusurinaria induces apoptosis in human cancer cells. Am J Chin Med., 2004; 32: 175-183.
- Joseph, S.R. An overview: Pharmacognostic properties of Phyllanthus aramurus Int. J. Pharmacol., 2011; 7: 40-45.
- Nguyen, T.H.K., Doan, T.T.V., Ha, D.L., Nguyen, N.H. Molecular cloning, expression of minD gene from Lactobacillus acidophilus VTCC-B-871 and analyses to identify Lactobacillus rhamosus PN04 from Vietnam Hottuyniacordata Indian J. Microbiol., 2013; 53: 385-390.
- Niggemann, B., Gruber, C. Side-effects of complementary and alternative medicine. , 2003; 58: 707-716.
- Nordwig, S.T., Birger, K., Elisabeth, A. Staphylococcal toxins in patients with psoriasis, atopic dermatitis, and erythroderma, and in healthy control subjects.J. Am. Acad. Dermatol.2005; 53(1): 67-72.
- Oluwafemi,, Debiri, F. Antimicrobial effect of Phyllanthus amarus and Parquetina nigrescens on Salmonella typhi. Afr. J. Biomed. Res., 2008; 1: 215 – 219.
- Richard R. M., Charles, L.H., Albert, M.K. Staphylococcus aureus in Psoriasis. Arch Dermatol.,1973; 107(4):568-570.







