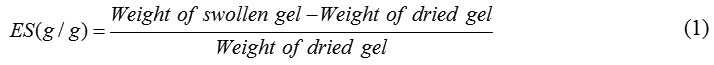Synthesis of a Novel Pectin-Based Superabsorbent Hydrogel with Salt and pH-Responsiveness Properties
B. Kazemzadeh1, H. Hosseinzadeh2* and M. Babazadeh1
1Department of Chemistry, Science Faculty, Islamic Azad University, Tabriz Branch, Tabriz, Iran. 2Department of Chemistry, Payame Noor University, 19395-4697, Tehran, Iran.
Corresponding Author E-mail: h_hoseinzadeh@pnu.ac.ir
Abstract
A novel smart superabsorbent hydrogel with salt- and pH-responsiveness properties was obtained by grafting of mixtures of acrylic acid (AA) and acryl amide (AM) monomers onto pectin, using ammonium persulfate (APS) as a free radical initiator in the presence of methylene bisacrylamide (MBA) as a crosslinker. The graft copolymerization conditions were systematically optimized to achieve a hydrogel with swelling capacity as high as possible. Infrared spectroscopy was carried out to confirm the chemical structure of the hydrogel. Moreover, morphology of the samples was examined by scanning electron microscopy (SEM) and thermogravimetric analysis TGA. Results from SEM observation also showed a porous structure with smooth surface morphology of the hydrogel. The swelling variations of hydrogels were explained according to swelling theory based on the hydrogel chemical structure. The hydrogels exhibited salt-sensitivity and cation exchange properties. The pH-reversibility property of the hydrogels makes the intelligent polymers as good candidates for considering as potential carriers for bioactive agents, e.g. drugs.
Keywords
Superabsorbent; pectin; acrylic acid; acryl amide; hydrogel
Download this article as:| Copy the following to cite this article: Kazemzadeh B, Hosseinzadeh H, Babazadeh M. Synthesis of a Novel Pectin-Based Superabsorbent Hydrogel with Salt and pH-Responsiveness Properties. Biomed Pharmacol J 2013;6(1) |
| Copy the following to cite this URL: Kazemzadeh B, Hosseinzadeh H, Babazadeh M. Synthesis of a Novel Pectin-Based Superabsorbent Hydrogel with Salt and pH-Responsiveness Properties. Biomed Pharmacol J 2013;6(1). Available from: http://biomedpharmajournal.org/?p=2606 |
Introduction
In recent years, much interest has been shown in the development of synthesis of natural-based superabsorbent hydrogels [1-4]. Superabsorbent polymers (SAPs) are defined as hydrophilic, three-dimensional networks with the ability of absorbing large values of water, saline solutions, or physiological fluids [5-6]. They are widely used in various applications such as hygienic, foods, cosmetics, and agriculture [7-9]. In general, the properties of the swelling medium (e.g. pH, temperature, and ionic strength) affect the swelling characteristics. SAPs responding to external stimuli such as heat, pH, electric field, chemical environments, etc, are often referred to as “intelligent” or “smart” polymers.
Synthesis and investigation of specific and new superabsorbent hydrogels with high absorbency, mechanical strength and initial absorption rate, has been the goal of several research groups in the past decades. A number of such biomaterials have been prepared by free radical graft copolymerization of vinylic monomers onto polysaccharides. Indeed, vinyl graft copolymerization onto polysaccharides and proteins is a well-known method for synthesis of natural-based superabsorbent hydrogels [10-12]. The first industrial superabsorbent hydrogel, hydrolyzed starch-graft-polyacrylonitrile, was synthesized using this method [13].
The presence of the natural parts guarantees biocompatibility, biodegradability, and non-toxicity of the superabsorbing materials. Therefore, in this work, therefore, we attempted to synthesize and investigate the swelling behavior of a novel superabsorbing hydrogel from pectin-g-poly (acrylic acid-co-acryl amide). The reaction variables affecting the water absorbency of the hydrogel as well as the salt-, pH-, and temperature sensitivity of the hydrogels were investigated in detail.
Experimental part
Materials
The polysaccharide, pectin, (from Fluka), N,N’-methylene bisacrylamide (MBA, from Merck), ammonium persulfate (APS, from Fluka), and acrylamide (AM, from Merck) were of analytical grade and used without further purification. Acrylic acid (AA from Merck) as ionic monomer was used after vacuum distillation for removing inhibitor. Distillate water was used for the hydrogel preparation and swelling measurements.
Preparation of hydrogel
Certain amounts of distilled water (30 mL) and pectin (2.0 g), were added to a three-neck reactor equipped with a mechanical stirrer (Heidolph RZR 2021), while stirring (500 rpm). The reactor was placed in a thermostated water bath preset at 80oC for 20 min. After dissolving pectin and homogenizing the mixture, the monomers, AA and AM, and the crosslinker, MBA, were simultaneously added and the reaction mixture was stirred for 20 min. Then, the APS initiator was added and gelation was observed after 30 min. After 1h, the mixture was treated with 1 N sodium hydroxide for 70% neutralization of the carboxylic groups of the grafted poly (acrylic acid). Finally, the gel product was poured into 100 mL of ethanol for 2 h and then scissored to small pieces. The non-solvent ethanol was then decanted and 100 mL fresh ethanol was added. The particles were remained for 24 h to completely solidify. The dewatered gel particles were filtered and dried in oven at 45 oC for 6 h. After grinding, the powdered superabsorbent hydrogel was stored away from moisture, heat and light.
Swelling measurements
An accurately weighed sample (0.2 ± 0.001 g) of the powdered superabsorbent with average particle sizes between 40-60 mesh (250–350) was immersed in distilled water (200 mL) and allowed to soak for 3 h at room temperature. The equilibrium swelling (ES) capacity was measured twice at room temperature according to a conventional tea bag (i.e. a 100 mesh nylon screen) method using the following formula:
Swelling in various salt solutions
Absorbency of the pectin-g-poly(AA-co-AM) hydrogel sample was evaluated in 0.15 M solutions of various salts according to the above method described for swelling measurement in distilled water.
Instrumental analysis
Fourier transform infrared (FTIR) spectroscopy absorption spectra of samples were taken in KBr pellets, using an ABB Bomem MB-100 FTIR spectrophotometer (Quebec, Canada), at room temperature. The surface morphology of the gel was examined using scanning electron microscopy (SEM). After Soxhlet extraction with methanol for 24 h and drying in an oven, superabsorbent powder was coated with a thin layer of gold and imaged in a SEM instrument (Leo, 1455 VP). Thermogravimetric analyses (TGA) were performed on a Universal V4.1D TA Instruments (SDT Q600) with 8–10 mg samples on a platinum pan under nitrogen atmosphere. Experiments were performed at a heating rate of 20 oC/min until 600 oC.
Results and discussion
Preparation of hydrogel
Scheme 1 shows a simple structural proposal of the graft copolymerization of acrylic acid (AA) and acryl amide (AM) monomers onto the pectin backbones and crosslinking of the graft copolymer. The thermally dissociating initiator, i.e. APS, is first decomposed under heating (80 oC) to produce sulphate anion-radicals. Then, the anion-radicals abstract hydrogen from the pectin backbones is used to form corresponding macroinitiators. These macroradicals initiate grafting of AA and AM onto pectin backbones leading to a graft copolymer. Crosslinking reaction also occurred in the presence of the crosslinker, i.e. MBA.
 |
Scheme 1: Mechanistic pathway for synthesis of pectin-g-poly(AA-co-AM) hydrogel.
|
Spectral Characterization
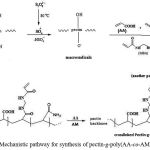
For identification of the hydrogel, infrared spectroscopy was used. Figure 1 shows the IR spectroscopy of pectin (A), poly(AA-co-AM) (B) and pectin-g-poly(AA-co-AM) hydrogel (C). The superabsorbent hydrogel product comprises a pectin backbone with side chains that carry sodium carboxylate and carboxamide functional groups that are evidenced by peaks at 1550 and 1670 cm-1, respectively. The intense characteristic band at 1550 cm-1 is due to C=O asymmetric stretching in carboxylate anion that is reconfirmed by another sharp peak at 1415 cm-1 which is related to the symmetric stretching mode of the carboxylate anion.
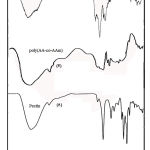 |
Figure 1: FTIR spectra of pectin (A), poly(AA-co-AM) (B) and pectin-g-poly(AA-co-AM) hydrogel (C).
|
To obtain additional evidence of grafting, a similar polymerization was conducted in the absence of the crosslinker. After extracting the homopolymer and unreacted monomers using a cellophane membrane dialysis bag (D9402, Sigma–Aldrich), an appreciable amount of grafted pectin (85%) was observed. The graft copolymer spectrum was very similar to Figure 1-C. According to the preliminary measurements, also, the sol (soluble) content of the hydrogel networks was as little as 1.9 %. This fact, practically, proves that all monomers are almost involved in the polymer network. So, the monomers’ percent in the network will be very similar to that of the initial feed of reaction.
Scanning electron microscopy
One of the most important properties that must be considered is hydrogel microstructure morphologies. The surface morphology of the samples was investigated by scanning electron microscopy. Figure 2 shows an SEM micrograph of pectin and the polymeric hydrogels obtained from the fracture surface. The hydrogel has a porous structure. It is supposed that these pores are the regions of water permeation and interaction sites of external stimuli with the hydrophilic groups of the graft copolymers.
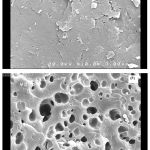 |
Figure 2: SEM photograph of pectin (A) and hydrogel (B).
|
Thermogravimetric analysis
Figure 3 shows TGA of pectin (Fig. 3A) and the hydrogels (Fig. 3B). Comparison of these curves indicates that the structure of pectin chains has been changed, which might be due to the grafting of PAA and PAM chains. In general, the hydrogel had lower weight loss than pectin. This means that the grafting of pectin increases the thermal stability of pectin in some extent. Indeed, with introducing of PAA and PAM chains on pectin backbones the maximum of degradation speed in pectin (285 oC) was shifted to 342 oC.
 |
Figure 3: TGA thermograms of (A) pectin and (B) pectin-g-poly(AA-co-AM) hydrogel.
|
Investigation of effect parameters onto water absorption
In this work, the main factors affecting on the swelling, i.e. concentration of pectin, MBA, AA/AM ratio, and APS, salinity and pH-sensitivity of the hydrogel were systematically optimized to achieve superabsorbent with maximum water absorbency.
Effect of pectin concentration
The swelling dependency on pectin amount is shown in Figure 4. Maximum swelling (61 g/g) has been observed at 0.75 g of pectin, while other factors including monomer, initiator, MBA, reaction time and temperature were kept constant. Swelling of hydrogel is considerably increased with increasing of pectin value from 0.25 to 00.75 g. This behaviour is attributed to the availability of more grafting sites for initiation of graft copolymerization at higher pectin concentration. However, upon further increase in the substrate concentration, increase in the reaction medium viscosity restricts the movements of macroradicals, thereby decreasing the grafting ratio and consequent decreasing the absorbency. It also may be attributed to deactivation of the macroradical growing chains (e.g., by transfer reactions, combination and/or interaction with the primary radicals) soon after their formation.
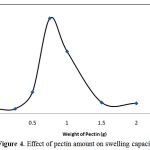 |
Figure 4: Effect of pectin amount on swelling capacity.
|
Effect of MBA concentration on swelling capacity
The effect of crosslinker concentration on swelling capacity of pectin-g-poly(AA-co-AM) was investigated. As shown in Figure 5, more values of absorbency are obtained by lower MBA concentration as reported by pioneering scientists [14]. In fact, higher crosslinker concentrations decrease the free space between the copolymer chains and consequently the resulted highly crosslinked rigid structure can not be expanded and hold a large quantity of water. The maximum absorbency (200 g/g) is achieved at 0.05 g of MBA.
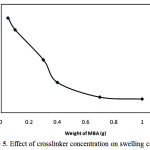 |
Figure 5: Effect of crosslinker concentration on swelling capacity.
|
Effect of monomer ratio on swelling capacity
The swelling capacity of the hydrogels, prepared with various ratios of monomers, is shown in Figure 6. The presence of the ionic groups in polymer chains results in increasing of swelling because the ions are more strongly solvated rather than non-ionic groups in the aqueous medium. Therefore, the swelling enhancement versus higher AA/AM ratio can be attributed to the formation of high carboxylate groups.
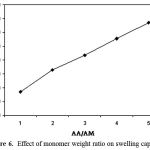 |
Figure 6: Effect of monomer weight ratio on swelling capacity.
|
Effect of initiator concentration
For studying the initiator concentration effect on absorbency, amount of APS from 0.05 to 0.5 g was varied (Fig. 7). The absorbency is increased versus increasing the APS concentration from 0.05 up to 0.1 g and then, it is decreased considerably with a further increase in the amount of APS. The maximum absorbency (244 g/g) is obtained at APS 0.1 g. The number of active free radicals on the pectin backbone is increased in terms of the initiator levels lower than 0.1 g. This accounts for the initial increment in swelling up to a certain amount of APS. The swelling decrease after the maximum may be attributed to increased number of produced radicals led to terminating step via bimolecular collision resulting in enhanced crosslink density. This possible phenomenon is referred to as “self crosslinking”. An additional reason for decreasing the absorbency can be related to decreasing molecular weight (MW) of the grafted PAA and PAM at high levels of APS concentration. Since MW inversely depends on initiator concentration, [I], higher [I] results in lower MW and, in turn, lower swelling capacity of the hydrogel.
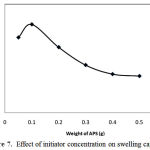 |
Figure 7: Effect of initiator concentration on swelling capacity.
|
Effect of water temperature
Figure 8 shows the effect of temperature of swelling medium on water absorbency. As shown in this figure, with initial increasing the temperature the swelling capacity was increased because of higher motions of water molecules. The swelling decrease with higher increase in temperature may be attributed to chain degradations of hydrogel backbones.
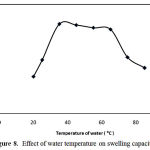 |
Figure 8: Effect of water temperature on swelling capacity.
|
Salt-sensitivity of pectin-g-poly(AA-co-NIPAM) hydrogel
Swelling capacity in salt solutions is of prime significance in many practical applications such as personal hygiene products and water release systems in agriculture. The swelling ability of “anionic” hydrogels in various salt solutions is appreciably decreased compared to the swelling values in distilled water. This well-known undesired swelling-loss is often attributed to a “charge screening effect” of the additional cations causing a non-perfect anion–anion electrostatic repulsion [14]. In salt solution, also, the osmotic pressure resulting from the difference in the mobile ion concentration between gel and the aqueous phases is decreased and consequently the absorbency amounts are diminished. In addition, in the case of salt solutions with multivalent cations, “ionic crosslinking” at surface of hydrogel particles causing an appreciably decrease in swelling capacity.
Since the pectin-based hydrogels are comprised poly(NaAA) chains with carboxylate groups that can interact with cations, they exhibit various swelling capacity in different salt solutions with same concentrations (Figure 9). In the presence of the bivalent and trivalent ions, the crosslinking density increases because of interaction of these ions with carboxylate groups leading to “ionic crosslinking”.
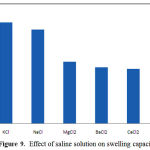 |
Figure 9: Effect of saline solution on swelling capacity.
|
The swelling–deswelling cycle of the hydrogel in sodium and calcium salts are shown in Figure 10. In sodium solution, swelling of the hydrogel is increased with time. When this hydrogel is immersed in calcium chloride solution, it deswells to a collapsed form. When the shrinked hydrogel is immersed in sodium chloride solution again, the calcium ions are replaced by sodium ions. This ion exchange disrupts the ionic crosslinks leading to swelling enhancement. When hydrogel is treated alternatively with NaCl and CaCl2 solutions with equal morality the swelling reversibility of hydrogel is observed.
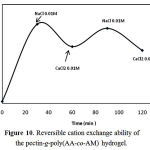 |
Figure 10: Reversible cation exchange ability of the pectin-g-poly(AA-co-AM) hydrogel.
|
pH-responsiveness behaviour of the hydrogel
Ionic superabsorbent hydrogels exhibit swelling changes at a wide range of pHs. Swelling of the synthesized hydrogels was measured in solutions buffered at various pH values, from 3.0 to 12.0. The solutions were composed sodium hydroxide and phosphoric acid. Results are shown in Figure 11. A maximum swelling at pH 8 (71 g/g) is obvious from the figure. The reasons of the swelling loss for the highly acidic and basic solutions are “charge screening effect” as the same described formerly.
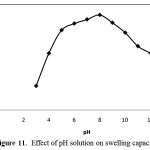 |
Figure 11: Effect of pH solution on swelling capacity.
|
We also investigated the reversible swelling-deswelling behavior of this hydrogel in solutions with pH 2.0 and 8.0 (Figure 12). At pH 8.0, the hydrogel swells due to anion-anion repulsive electrostatic forces, while at pH 2.0, it shrinks within a few minutes due to protonation of the carboxylate anions. This swelling-deswelling behavior of the hydrogels makes them as suitable candidate for designing drug delivery systems.
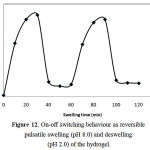 |
Figure 12: On-off switching behaviour as reversible pulsatile swelling (pH 8.0) and deswelling (pH 2.0) of the hydrogel.
|
Conclusions
A novel biopolymer-based superabsorbent hydrogel, pectin-g-poly(AA-co-AM) was synthesized through simultaneous crosslinking and graft polymerization of acrylic acid/acrylamide mixtures onto pectin. In order to prove that AA and AM molecules were grafted, FTIR and SEM spectroscopes and TGA analysis were used.
Swelling capacity of the hydrogels is affected by factors such as the crosslinker (MBA) concentration and monomer ratio, so that the swelling is decreased by increasing the MBA concentration and AM/AA ratio. The swelling capacity in CaCl2 is much lower than that in NaCl solution and distillated water. The swelling–deswelling process of the hydrogel alternatively carried out in CaCl2 and NaCl solutions results in a high capability of ion exchanging of the pectin-based hydrogel.
The swelling of hydrogel exhibited high sensitivity to pH study of the effect of H+/OH– concentration which was carried out at various pHs shows that the swelling of hydrogel causes several large volume changes. So, we investigated the pH-sensitivity of the hydrogel. Ionic repulsion between charges groups incorporated in the gel matrix by an external pH modulation could be assumed as the main driving force responsible for such abrupt swelling changes. This superabsorbent network intelligently responding to pH may be considered as an excellent candidate to design novel drug delivery systems.
References
- Wang W, Wang A. Carbohydr Polym 2010; 80: 1028.
- Zhou H. Y., Chen XG, Kong M, Liu CS. J Appl Polym Sci 2009; 112: 1509.
- Sadeghi M, Hosseinzadeh H. Turk J Chem 2008; 32: 375.
- Chatterjee S, Chatterjee T, Woo SH. Bioresource Technology 2010; 101: 3853.
- F. L. Buchholz, and A. T. Graham, Modern Superabsorbent Polymer Technology, Elsevier: Amsterdam (1997).
- L. B. Peppas, and R. S. Harland, Absorbent Polymer Technology, Elsevier: Amsterdam (1990).
- R. Po, Journal of Macromolecular Science-Reviews in Macromolecular Chemistry and Physics, C34, 607 (1994).
- N. A. Peppas, and A. G. Mikes, Hydrogels in Medicine and Pharmacy, vol.1, CRC Press: Boca Raton, FL, (1986).
- A. S. Hoffman, Polymeric Materials Encyclopedia, Salamone, J. C. (Ed.), p. 3282, CRC Press: Boca Raton, FL, (1996).
- M. Yazdani-Pedram, J. Retuert, and R. Quijada, Macromolecular Chemistry and Physics, 201, 923 (2000).
- Y. Sugahara, O. Takahisa, Journal of Applied Polymer Science, 82, 1437 (2001).
- G, M. Patel, and H. C. Trivedi, European Polymer Journal, 35, 201 (1999).
- G. F. Fanta, Polymeric Materials Encyclopedia, Salamone, J. C. (Ed.), CRC Press: Boca Raton, FL, vol.10, p. 7901 (1996).
- J. Flory, Principles of Polymer Chemistry, Ithaca, Cornell University Press, New York, (1953).