M.T. Jagannath Bose1, M. Ilavazhahan1, R. TamilselvI2 and M.Viswanathan1
1Department of Zoology, Sir Theagaraya College, Chennai - 600 021, India.
2Department of Chemistry, Bharathi Women’s College, Chennai - 600 108, India.
Corresponding Authors Email.jagannathbose@Ymail.com
Abstract
Histopathological effect of acute concentrations of heavy metals such as copper, ferroussulphate were studied. Results revealed extensive histopathological abnormalities in the internal structure of gill and brain of Catlacatla exposed to copper and ferrous sulphate in contrast to that of control fish, showing the severity of heavy metal toxicity.
Keywords
Catlacatla; Copper; Ferrous Sulphate; Histopathology; Gills; Brain
Download this article as:| Copy the following to cite this article: Bose M. T. J, Ilavazhahan M, TamilselvI R, Viswanathan M. Effect of Heavy Metals on the Histopathology of Gills and Brain of Fresh Water Fish Catla catla. Biomed Pharmacol J 2013;6(1) |
| Copy the following to cite this URL: Bose M. T. J, Ilavazhahan M, TamilselvI R, Viswanathan M. Effect of Heavy Metals on the Histopathology of Gills and Brain of Fresh Water Fish Catla catla. Biomed Pharmacol J 2013;6(1). Available from: http://biomedpharmajournal.org/?p=2632 |
Introduction
Pollution is an undesirable change in the physical, chemical or biological characteristics of our land, air or water that may or will harmfully affect human life or that of desirable species. India is rich in inland fisheries resource. But the indiscriminate use of heavy metals causes serious threat to such resources. Toxicity of a substance is known by its capacity to cause adverse effects on the living organisms. Toxic impact may bring about physiological, biochemical or pathological alterations in the organisms; the signs of toxicity may reveal symptoms of illness varying from simple local effects – structural and behavioral (Shivakumaret al., 2005) to complex disorders resulting in mortality.The intoxication includes a sequence of events that start with the exposure of a substance to an organism. Subsequently, the toxic substances are absorbed into the viscera by various routes causing an internal exposure (Tilaket al., 2005). Iron is the fourth most abundant, by weight, of the elements that make up the earth’s crust. Common in many rocks, it is an important component of many soils, especially clay soils where it is usually a major constituent.
The ferrous, or bivalent (Fe++), and the ferric, or trivalent (Fe+++) ions, are the primary forms of concern in the aquatic environment, although other forms may be in organic and inorganic wastewater streams. The ferrous (Fe++) form can persist in waters void of dissolved oxygen and originates usually from groundwater or mines when these are pumped or drained.
In nature, aquatic animals are constantly exposed to toxicants including metallic ions. Concentrations of metals in water are determined by geochemical processes and large scale release into the aquatic environment by human activities. According to Forstner and Wittman (1983) the harmful effects of heavy metals as pollutants result from incomplete biological degradation. Therefore, these metals tend to accumulate in the aquatic environment. Since heavy metals are non-biodegradable, they can be bio accumulated by fish, either directly from the surrounding water or by ingestion of food. Human population growth and industrial development have been the major causes of coastal contamination around the world during recent years (Caussyet al., 2003). The subsequent accumulation of xenobiotic compounds in sediment, seawater or prey organisms has been shown to be directly linked to adverse health in both humans and fish (Ashraf, 2005).
Heavy metals are known to be highly toxic to most organisms and therefore, its input into aquatic medium is an important aspect of environmental pollution. In most waters, the concentrations of heavy metals are very low but aquatic systems associated with out cropping metalliferous loads contain higher concentration of heavy metals. Among the heavy metals, cadmium, lead, mercury, copper, zinc, chromium and nickel are comparatively notorious toxicants and most of their compounds are water soluble and non–degradable. Copper and Iron are the heavy metals, which are used extensively in printing, oil industries, dying industries, vulcanization of rubber and in ore extraction. Copper has been used for many years as a chemical tool in freshwater farm ponds as an effective algaecide and for parasitic treatment. Copper is an essential element needed for many physiological processes, serving as enzyme activators. But at high concentrations, copper is toxic. Fish are exposed to copper, both in the diet and the external environment.
Exposure to copper can induce stress responses: changes in the fish’s ion regulation, olfaction and swimming performance (Boecket al., 2003 and Naskaret al., 2004). At certain concentrations, iron can also be toxic to aquatic life. The most common form of iron in solution in anoxic groundwater is the ferrous ion. Soluble ferrous iron typically enters surface waters from ground waters or mines (or waste deposits and tailings) when they are pumped or drained. Ferrous iron can also be found in the deep waters of stratified lakes with anaerobic hypolimnia. The changes brought about by the stressors may be symptomatic or asymptomatic with signs of changes in the behavior, structural or morphological abnormalities, alterations in the biochemical and physiological profiles or changes at cellular level in the different tissues.
Histopathological investigations have proved to be a sensitive tool to detect direct effects of chemical compounds within target organs of fish in laboratory experiments. The degree of pathological intensity is dependent on the dose and duration of exposure. Histopathological studies have been conducted to help establish causal relationships between contaminant exposure and various biological responses. Histopathological biomarkers embody tissue lesions arising as a result of a previous or current exposure of the organism to one or more toxins.
This type of study in fish has been to a great extent is handicapped because of the lack of adequate histological literature concerning various fish organs ( Hinton et al.,1972). Hence, the present study has been undertaken to examine the effect of copper and ferrous sulphate on the histopathological changes of gills and brain of Catlacatla.
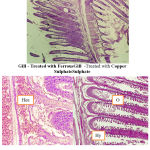 |
Figure 1: Gill – Treated with FerrousGill -Treated with Copper SulphateSulphate
|
Circulatory anomalies and haemorrhage
hyperplasia
Oedema
Materials and Methods
Fingerlings of Catlacatla of relatively same size ranging from (8 to10 cm) and weight about (5-8 gm) were collected from culture ponds of Bharath Fish Farm, Poondi, Thiruvallur district, Tamil Nadu. The fish were oxygen packed in polythene bags and brought to the laboratory with minimal stress during transit. They were released very carefully into the fish tanks half filled with bore well water. They were maintained in the stocking tank and acclimatized before experimentation.
The fish feed was prepared with sieved rice bran, pounded groundnut oil cake, tapioca powder and mineral mixture as followed by Ramaiah (1982). Groundnut oil cake was soaked in distilled water and minced thoroughly before mixing with rice bran, tapioca powder and mineral mixture. The contents after mixing were steam cooked in autoclave at 20 psi for 15 minutes. Pellets were prepared and shade dried at room temperature for 24 hr and later in hot air oven at 60º C for 48 hr. The pellets were stored in air tight containers.The fish fingerlings were fed daily with pelleted feed at 5% body weight, in two split doses, one in the morning and the other in the evening. The feeding was stopped one day prior to experiment.
The fish without any structural, behavioral and clinical symptoms were chosen for experiments, after careful observation. Fish were divided into groups of ten each and exposed to heavy metal compound Ferroussulphate and a copper sulphateindependently. Preliminary studies were carried out to find out the median lethal concentration LC50 for 24 hour of copper and ferrous to Catlacatla according to the method of Finney, 1978. Fish were then exposed to acute toxicity of the above mentioned metals along with the control fish. At the end of 24 hour, fish from control and experimental medium were sacrificed and gills and brain gently separated for histopathological studies.
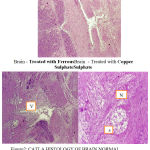 |
Figure 2: Brain – Treated with FerrousBrain – Treated with Copper SulphateSulphate
|
Vacuolization
Atrophy
Necrosis
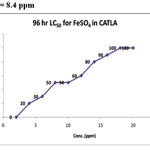 |
Figure 3: LC50 = 8.4 ppm
|
Results and Discussion
To determine LC50 for the different groups based on the cumulative percentage mortality at the end of 96hr of experimentation the standard graphic method was followed. The data was subjected to Probit analysis and slope function test to assess the upper and lower confidence limits. One fourth of LC50 values obtained from the above experiments was taken as the sub lethal concentration (SLC). The highest mortality of 100% was observed at 13ppm and 15ppm concentrations. 50% mortality was recorded in 8 and 9ppm concentration and 50% of mortality line intercepted the graph to show 8.4ppm as corresponding LC50 for the toxicant. SLC was calculated as 2.1ppm from the 96hr LC50 value(Table.1). On analysis of LC50 by arithmetic graphic method, it was found to be 4.8ppm, which was taken as 96hr LC50 for copper toxicity. SLC was calculated as 1.2ppm being 1/4th of LC50 value(Table.2).
Table 1: Determination Of 96 Hr Lc50 for Ferrous Sulphate in Catla
| S.No. | Conc. (ppm) | PERCENTAGE MORTALITY | CUMULATIVE % MORTALITY | |||
| DAY 1 | DAY 2 | DAY 3 | DAY 4 | |||
| 1 | 2 | 0 | 0 | 0 | 0 | 0 |
| 2 | 4 | 0 | 0 | 10 | 10 | 20 |
| 3 | 6 | 0 | 0 | 10 | 20 | 30 |
| 4 | 8 | 0 | 10 | 20 | 10 | 50 |
| 5 | 10 | 10 | 10 | 10 | 20 | 50 |
| 6 | 12 | 10 | 20 | 20 | 0 | 60 |
| 7 | 14 | 10 | 10 | 20 | 30 | 80 |
| 8 | 16 | 10 | 20 | 30 | 30 | 90 |
| 9 | 18 | 20 | 30 | 40 | 0 | 100 |
| 10 | 20 | 40 | 50 | 10 | 0 | 100 |
Table 2: Determination Of 96 Hr Lc50 for Coppersulphate in Catla
| Conc. (ppm) | PERCENTAGE MORTALITY | CUMULATIVE % MORTALITY | |||
| DAY
1 |
DAY
2 |
DAY
3 |
DAY
4 |
||
| 2 | 0 | 0 | 0 | 1 |
10
|
| 4 | 0 | 1 | 5 | 2 |
80
|
| 6 | 1 | 3 | 5 | 0 |
90
|
| 8 | 1 | 8 | 0 | 0 |
90
|
| 10 | 10 | 0 | 0 | 0 |
100
|
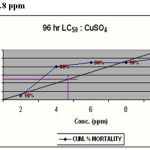 |
Figure 4: LC50 = 8.4 ppm
|
In the present study the fish showed characteristic change in behavior when transferred to experimental chambers having different mental. The fishes survived rapidly in the experimental media and all the fish showed swirling movement, swimming on long axis, and frequent surfacing with rapid opercular beat during the 6hr period post inoculation. 30% developed dropsical symptom which increased to 100% in 48hr. Reddening at the lateral sides of the belly was observed in 20% of the fish. Morbidity developed in 30% of the fish which exhibited feeble opercular beat and the fish tended to lie at the bottom on its lateral side. The fish showed uncoordinated swimming activity, loss of reflexes, loss of balance and erratic swimming activity with occasional darting movement. During the experimentation period, 30% of the fish showed feeble opercular movement or floated upside down gasping and struggling to breath, which is in apparent with the studies of Loharet al.,)2000) and Mubarak begam, (1998) and AnithaKumari, (1998).
The histopathological observations were made from gill, brain of the control and experimental groups which were exposed to the toxicants for 24hrunder independent toxicity experiments. Fish were killed and the tissues (gill and brain) were fixed in 10% neutral buffered formalin. The fixed tissues were dehydrated in an increasing gradient of alcohol (70, 80, 90, and 100%) for 30 min each and were eventually dried in acetone, and cleared in xylene for 30min. The tissues were then infiltrated by embedding in molten wax and sectioned at 8µ. The paraffin sections were then mounted on a slide, stained with haematoxylin and counterstained with eosin.
To study the pathogenicity of various toxicants, the section of tissues were observed under microscope and the conditions in different tissues were photographed at lower and higher power of magnification using Nikon micro photographic equipment.
No fish died during the acclimation period before toxicant exposure, and no fish died during toxicity tests. Tissue sections of organs like brain and gill of catla fingerlings treated with bothtoxicants independentlyare observed for histopathological conditions. In fish, gill is the first organ to which any pollutant comes into contact. Fish gill is very sensitive to changes in the composition of the environment and is an important indicator of waterborne toxicants. Consequently, injury to gill epithelium is a common response observed in fish exposed to a variety of contaminants.
The severity of damage to the gills depends on the concentration of the toxicant and the period of exposure.The gill arches of Catlacatlain the control group showed normal arrangement pattern of primary and secondary lamellae. The arches contain primary lamellae. Projecting on the lateral sides of primary lamellae are the secondary lamellae. The entire mass of primary lamellae is covered by stratified squamous epithelium. The surface of the secondary lamellae is covered with a delicate layer of a simple squamous epithelium that is the active exchange pillar cells. In the core of the primary lamellae is a rigid mass of cartilaginous tissues around which are traces of vascular channels. The chloride cells are more frequent at the base of the secondary lamellae.
Varied morphological changes occurred in the gill tissue of the treated fingerlings of catlaand the gill exhibited marked alterations in their epithelia. The epithelium was no longer continuous, particularly the more delicate respiratory lamellae. Thus, there was fusion at adjacent secondary lamellae as a result of hyperplasia. Oedema at the secondary lamellae and swelling of the epithelia cells were observed. The pillar cells have been altered and blood spaces expanded. There is a severe hyperplasia with profound oedematous changes characterized by epithelial detachment. The elongated secondary lamellae with club-like structures were the other pathological changes observed in the histological sections of gills of the fingerlings of catla treated with two toxicants.
The nutritional gill disease consists of lamellar epithelial hyperplasia with eventual fusion of secondary lamellae near the tips of gill filaments (Lohar, 2000). Circulatory anomalies and haemorrhage were also manifested in affected gills with an indication by severe congestion of blood spaces by erythrocytes. Sometimes presence of leukocytes particularly in the lamellae can be together with the presence of mucin, depicting an inflammatory reaction (Elahee and Bhagwant, 2006).Histological evidence in the present study is in conformity with the work ofVeenaSakthivel and Gaikwad (2002). Brain revealed generalized congestion and dilation of meningeal vessels along with infiltration of mononuclear cells. The major histological changes, observed in the toxicant treated brain of the fish were degeneration of granular and molecular layer. Vacuolization and necrosis of the brain cells was observed. Other pathological changes observed in the brain of exposed fish include degeneration of nerve cells, atrophy, dissolution of nissel bodies, swelling of the axon, and cellular damage in the interior and posterior regions.
The major histological changes observed in the heavy metals treated brain of the fish showed degeneration of granular and molecular layer, cellular damage in the interior and posterior regions of the brain, degeneration of the nerve cells, vacuolization and necrosis of the brain cells. Anita Kumari (1998) have made similar observations which are in conformity with the present study on the brain of Catlacatla.
Brain revealed generalized congestion and dilation of meningeal vessels along with infiltration of mononuclear cells. Other pathological changes observed in the brain of exposed fish include atrophy, necrosis and dissolution of nissel bodies, swelling of the axon and vacuolization of the myelin sheath of the nerve fibres.
Histopathological changes in the brain are characterized by vacuolation of brain parenchyma and moderate swelling of pyramidal cells of the cerebrum. Vacuolation may have been due to glycolysis leading to micorsomal and mitochondrial dysfunctions. Loss of Nissl substances and glial cell reaction, with evidence of glial nodule formation in places, was proof of the neurotoxic nature of the chemical.
From the above observations it is clear that the gills are more affected than the brain. Among the two heavy metals studied the effect of copper on the gills and the brain is more pronounced when compared to ferrous.The results of the present study point to the fact that the toxins of pollutants definitely affect the aquatic life of the fresh water fish. The problem can be more serious in the fish culture farms as the culture ponds are invariably located in or near the agricultural land, which were already loaded with toxic residues of all kinds. Added to this if the ground water used is also equally polluted with contaminants and pathogenic flora and fauna, the problem becomes compounded, with simulated experimental conditions as in the laboratory. Hence, a scientific method of detoxification is essential to improve the health of these economically important fish and reduce the losses caused by anthropogenic stress.
References
- AnithaKumari, S. Effect of water pollution on LDH isoenzymes in fish from HussainSagar Lake, Hyderabad. A.P. Ph.D thesis submitted. The Osmania University. Hyderabad (1998).
- Ashraf, M. 2005. Accumulations of heavy metals in kidney and heart tissues of Epinephelusmicrodon fish from the Arabian. Gulf. Environ. Monit. Asses., 101:311-316.
- Boeck(DR) G.Dewachter B.,Ulaeminck, A. and R. Blust. Effect of cortisla treatment and a sublethal copper exposure on copper uptake and heat shock protein levels in common carp cyprinuscarpio. Envirn. Toxi. Chem. 22:1122-1126 (2003).
- Caussy, D, Gochfeld, M. and E. Gurzau. 2003. Lesions from case studies of metals investigating exposure, bioavailability, and risk. Environ. Safety. 56:45-51.
- Elahee, K. B. and S. Bhagwant. Hematological and gill histopathological parameters of three Tropical fish species from a polluted Lagoon on the West Coast of Mauritius. And Environ. Safety. 1-11(2006).
- J. In. Statistical method in Biological Assay. 3rd Edi. Griffin Press, London, UK (1978).
- Forstner,U and G.T.W.wittmann. Metal pollution in the agnatic environment. 2nd Springer Vertag, New York. P486 (1983).
- Hinton, D.E., R.L.Snipes and M.L. Kendall.(1972). As citied by Joshi A.G. (1978).
- Lohar, P.S. Comparative toxicity of four heavy metals in freshwater fishes. Aqua. Biol. 15(1/2):95-98 (2000).
- Naskar, Nisarga.S, sen and M. Firoz Ahmad. Physiological response of an air breathing teleost, Clariasbatrachus(Linn.) to sub lethal Aluminium toxicity in acidic soft water. Zool. Soc. Calcutta. 57(1):29-34 (2004).
- Ramaiah, N. Bacterial diseases of Indian Major Carps and effects of chemotherapeutic drugs on the host and pathogen. M.F.Sc., Thesis. Coll. Fish., Mangalore (1982).
- Shivakumar, R. Mushigeri, S. B. and M. Davie. Effect of Endosulfan to freshwater fish Ctenopharyngodonidellus. Ecotoxl. Environ. Monit. 15:113-116(2005).
- Tilak, K.S., Veeraiah, K.andKoteswaraRao. Histopathological changes observed in the Gill, Liver, Brain and Kidney of the Indian Major Carp Cirrhinusmrigalaexposed to Chlorphrifos. Res., 24(1):101-111(2005).
- VeenaSakthivel and S.A. Gaikward. Tissue histopathology of Gambusiaaffinis (Baid and Giard) under dimecron toxicity. Ecol.Env. and Cons. 8(1):27-33 (2002).







