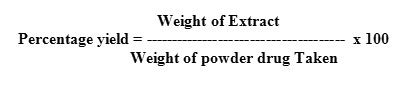Govind Patidar* and Anis Shaikh
Institute of pharmacy, Vikram University, Ujjain, India.
Abstract
The word ‘stress’ is defined as “a state of affair involving demand on physical or mental energy.” Stress is a condition which can disturb the normal physiological and psychological functions of an individual. In medical parlance ‘stress’ is defined as a perturbation of the body’s homeostasis. This demand on mind-body occurs when it tries to cope with incessant changes in life. Extreme stress conditions, psychologists say, are detrimental to human health but in moderation stress is normal and, in many cases, proves useful. Stress, nonetheless, is synonymous with negative conditions. During stressful situations the energy requirement of the organism is increased resulting in enhance generation of free radicals that causes oxidation of nucleic acid and proteins. Free radical also damage biomembrane, reflected by increased lipid peroxidation, thereby compromising cell integrity and function. During this process, the ability of the body’s defense system to combat the oxidative stress may diminish due to reduced antioxidants. Stress also increases brain serotonin (5-HT) level. The ascending 5-HT neurons from raphe nuclei innervates hypothalamic and limbic sites and have an overall role in regulating secretions of Adrenocorticotropic hormone (ACTH) during stress. The current research concludes that Glycyrrhizin at the doses of 100 and 200 mg/kg, p.o. reversed the behavioral and biochemical changes in Chronic Immobilization Stressed mice. So we can predict that Glycyrrhizin, the active constituent of liquorice shows antistress potential.
Keywords
Adrenocorticotropic hormone; Eustress; Hypostress; Antistress activity
Download this article as:| Copy the following to cite this article: Patidar G, Shaikh A. Antistress Potential of Glycyrrhizin in Chronic Immobilization Stress. Biomed Pharmacol J 2012;5(2) |
| Copy the following to cite this URL: Patidar G, Shaikh A. Antistress Potential of Glycyrrhizin in Chronic Immobilization Stress. Biomed Pharmacol J 2012;5(2). Available from: http://biomedpharmajournal.org/?p=2505 |
Introduction
The word ‘stress’ is defined as “a state of affair involving demand on physical or mental energy.” Stress is a condition which can disturb the normal physiological and psychological functions of an individual. In medical parlance ‘stress’ is defined as a perturbation of the body’s homeostasis. This demand on mind-body occurs when it tries to cope with incessant changes in life. Extreme stress conditions, psychologists say, are detrimental to human health but in moderation stress is normal and, in many cases, proves useful. Stress, nonetheless, is synonymous with negative conditions. During stressful situations the energy requirement of the organism is increased resulting in enhance generation of free radicals that causes oxidation of nucleic acid and proteins. Free radical also damage biomembrane, reflected by increased lipid peroxidation, thereby compromising cell integrity and function. During this process, the ability of the body’s defense system to combat the oxidative stress may diminish due to reduced antioxidants.2 Stress also increases brain serotonin (5-HT) level. The ascending 5-HT neurons from raphe nuclei innervates hypothalamic and limbic sites and have an overall role in regulating secretions of Adrenocorticotropic hormone (ACTH) during stress.1,2,3
Materials and Methods
Procurement and Identification of Crude Drug
The dried roots of glycyrrhiza glabra were procured from the market of Mandsaur (M.P.). The voucher specimen (BRNCP/Z/003/2010) was submitted in the department of Pharmacognosy, B. R. Nahata College of Pharmacy, Mandsaur (M.P.).
Isolation and Characterization of Glycyrrhizin
Isolation of Glycyrrhizin
Procedure
20g powdered drug was taken Added 20ml of Acetone 2ml of dil. HNO3 was added
Mixed, Corked the flask and Macerated for 2 hours with occasionally shaking Filtered the contents To the marc 20 ml of acetone was added, Warmed it on water bath and filtered Combined both filter and filtrate Added sufficient quantity of dil. Ammonia sol Precipitation of ammonium glycyrrhizin occurred Filtered the precipitate, washed it with 5ml of acetone twice Dried and weighed the product The yield of ammonium glycyrrhizin should be approximately 4.5% w/w.53
Determination of percentage yield
The percentage yield of extract was calculated by using following formula: –
Characterization of Glycyrrhizin
Physical Parameters
Following parameters were seen-
(a) Color
(b) Solubility
(c) Melting point
Chemical Test
Test for Liquorice
On addition with 80% sulphuric acid powdered drug showed
deep yellow color i.e. crude drug found to be liquorice.
Test for Saponin Glycoside
On addition and shaking with water glycyrrhizin
produced froth i.e. presence of triterpenoid saponin glycosides.54
Characterization Techniques
Following Techniques were used for the characterization of the glycyrrhizin.
(i) TLC (Thin layer chromatography)
(ii)UV-Spectroscopy
(iii) IR Spectroscopy
(i) TLC (Thin Layer Chromatography) –
TLC for Glycyrrhizin
Procedure
Applied small amount of each of test solution and standard solution in two
different tracks on a precoated silica gel plate. Developed the plate in the solvent system
to a distance of 12 cm.
Solvent system- Toluene: Ethyl acetate: Glacial acetic acid
Detection- (1) UV visible
(2) Anisaldehyde sulphuric acid reagent14
The Rf value of the spot was calculated using the formula-
Distance travelled by solvent
UV-Spectrophotometer
UV-Spectrophotometer is the best method for the identification of functional groups of unknown substances.55
IR Spectroscopy
IR is one of the most powerful analytical technique which offers the possibility of chemical identification and provides useful information about the structure of molecules.56
Pharmacological Study
Drug
Glycyrrhizin was isolated from Liquorice (unpeeled root) purchased from the local market of Mandsaur.
Animals
Male Albino mice weighing between 22-30 g of weight were obtained from B.R.N.C.P. Mandsaur Animal House. The animals were stabilized for 1 week; they were maintained in standard condition at room temp; 60 ± 5% relative humidity and 12 h light dark cycle. They had free access to food and water. Animals were acclimatized to laboratory conditions before the experiment. All the experiments were carried out between 09:00 and 15:00 h. The experimental protocols were approved by Institutional Animal Ethics Committee of B. R. Nahata College of Pharmacy, Mandsaur, (M.P.).
Chronic Immobilization Stress
The animals in all the groups except control (normal) were subjected to immobilization stress daily in a prone position for 150 min for 5 consecutive days using simple adhesive tape (chronic stress). Animals were released by removing the tape after moistening with acetone.57, 58
 |
Figure 1: Chronic Immobilization Stress in Mice.
|
2.3.4. Drugs and Treatment- Glycyrrhizin suspension was made by suspending glycyrrhizin in 1% CMC (Carboxy methyl cellulose) in distilled water. Fluoxetine solution was made by dissolving it in distilled water. Fluoxetine (10 mg/kg, i.p.) 59 was administered 30 min, Glycyrrhizin (100 and 200 mg/kg, p.o.) 36 and vehicle ( 1% CMC solution, p.o.) were administered 1 hour before subjected to chronic immobilized stress.
Groups
Group-I – Normal (Unstressed)
Group-II – Control (Stressed)q
Group-II – Glycyrrhizin (100mg/kg, p.o.)
Group-IV – Glycyrrhizin (200mg/kg, p.o.)
Group-V – Fluoxetine (10 mg/kg, i.p.) 36, 59
Behavioral Study
All the behavioral parameters were observed at the 6th day of chronic immobilization stress.
Measurement of Hyperalgesia
The hyperalgesia of animals were determined by Tail-flick method. In this method, the tip (last 1-2 cm) of the tail of animals were placed on the radiant heat source Analgesiometer). The tail withdrawal from the heat (flicking response) was taken as the end point (normal withdrawal time is 3-5 sec). A cut off period of 10-12 sec observed to prevent any damage to tail.58
Measurement of Anxiety
The anxiety level of various groups of mice was measured using mirror chamber and following parameters were recorded
(i) Latency to enter the chamber
(ii) Number of entries and time spent in mirror chamber
The mirror chamber consisted of a wooden chamber having a mirror enclosed within it.
Animal were placed individually at the distal corner of the mirror chamber at the beginning of the test.58, 6
Measurement of Locomotor activity
The locomotor activity was assessed using digital activity meter (Actophotometer). The
activity meter consisted of an arena (29x22x22 cm) and operated on photoelectric cells that were connected in circuit with a counter. When the animal cuts off the beam of light falling on photoelectric cell, a circuit was recorded. After subjecting mice to the stress and 30 minute after drug administration mice were placed gently in this arena and number of counts (locomotor activity scores) recorded for 10 minutes.58, 60
Measurement of Muscle co-ordination
Mice were subjected to motor function evaluation by placing them individually on Rota rod, which was adjusted to the speed of 25 rpm. The fall-off time was recorded for each mouse and the longest period any animal was kept on the rod was 300s.60
Measurement of Cognitive dysfunction
The Elevated plus maze served as the exteroceptive behavioral model to evaluate learning and memory in mice. The apparatus consisted of two open arms (16 cm X 5 cm) and two covered arms (16 cm X 5 cm X 12 cm) extending from a central platform (5 cm X 5 cm), which was elevated to a height of 25 cm from the floor. On the first day, each mouse was placed at the end of an open arm, facing away from the central platform. Transfer latency (TL) was taken as the time taken by the mouse to move into any one of the covered arms with all its four legs. TL was recorded on the first day. If the mouse did not enter into one of the covered arms within 90 sec, it was gently pushed into one of the two covered arms and the TL was assigned as 90 sec. The mouse was allowed to explore the maze for 10 sec and then was returned to its home cage. Memory retention was examined 24 h after the first day trial on the second day.61
Biochemical Parameters
On the 6th day of study, the animals were sacrificed by decapitation. The brains were removed, rinsed in isotonic saline and weighed. A 10% (w/v) tissue homogenate was prepared with 0.1 M phosphate buffer (pH 7.4). The post nuclear fraction for enzyme assay was obtained by centrifugation of the homogenate at 12,000 ×g for 20 min, at 4 °C.60
Measurement of Lipid peroxidation
Took 0.5 ml homogenate + 0.5 ml Tris HCL (PH- 7.4) and incubated at 370c for 2 hours Then 1 ml 10% TCA (Trichloro acetic acid) was added Centrifuged at 1000 x g for 10 minTo 1 ml supernatant, 1 ml of 0.67% TBA (Thiobarbituric acid) were added Kept the tubes in boiling water bath for 10 min Cooled the solution and added 1 ml of distilled water Absorbance measured at 532 nm using UV spectrophotometer values were expressed as nmol of malondialdehyde per mg protein
Estimation of Reduced Glutathione
(GSH) 1 ml of homogenate was precipitated with 1 ml of 4% sulfosalicyclic acid by keeping the mixture at 40c for 1 hour Immediately Centriguged at 1200 xg for 15 min Then 1 ml of supernatant, 0.2 ml of DTNB (Dithiobisnitrobenzoic acid) and 2.7 ml of phosphate buffer (0.1 M, PH-8) were taken The yellow color was measured at 412 nm using UV spectrophotometer value were expressed as nanomoles of reduced glutathione per mg of protein
Estimation of Nitrite
The accumulation of nitrite in the supernatant, an indicator of the production of nitric oxide (NO). Equal volumes of supernatant and Greiss reagent were mixed, the mixture was incubated for 10 min at room temperature in the dark and the absorbance at 540 nm was determined with UV spectrophotometer. The concentration of nitrite in the supernatant was determined from a sodium nitrite standard curve and expressed as micromoles nitrite per millimeter of homogenate.63
Protein Estimation
The protein content was measured according to the method of Lowry using bovine serum albumin as standard. In test tube 1ml of 1N NaOH solution was transferred and heated up to 100oc. Then suspended 1 ml of homogenate into the above solution for 5 minutes. Add 5 ml of alkaline copper reagent mix properly and leave the mixture at room temperature for10 min. Add 0.5 ml of Folin-ciocalteau reagent rapidly with immediate mixing. Leave it for 30 min., measure the absorbance of solution at 750 nm.64
Catalase Estimation
In this, we measured breakdown of hydrogen peroxide (H2O2) at 240 nm. Assay mixture consisted of 3ml of H2O2, phosphate buffer and 0.05 ml of supernatant of tissue homogenate (10%) and change in absorbance recorded at 240 nm. The result were expressed as micromole H2O2 decompose/mg of protein/min.65
Adrenal Ascorbic acid Estimation
The adrenal glands removed, rinsed in isotonic saline and weighed. A 1% (w/v) tissue homogenate was prepared with 0.1 M phosphate buffer (PH-7.4) and centrifuged at 12000 xg for 10 min, at 40c. The adrenal ascorbic acid levels were determined by 2, 4- dinitrophenyl hydrazine method. The value were expressed as microgram of ascorbic acid per mg of adrenal tissue.66
Statistical Analysis
The data were analyzed by Graph Pad Prism software demo version by one way analysis of variance (ANOVA) followed by “Dunnett’s test” and p value less than 0.05 were considered as statistically significant.
Result
Isolation And Characterization
Percentage yield
The percentage yield of Glycyrrhizin was found to be 4.2%.
Characterization of Glycyrrhizin
Following parameters showing the characterization of glycyrrhizin.
Physical parameters
Parameters Standard Test
Color
White to Brownish yellow powder Brownish yellow powder
Solubility
Freely soluble in hot water and alcohol, Sparingly soluble in hot water and alcohol
Melting point
2920C, 2850C
Chemical Test
Test for Glycosides – Positive
Test for Liquorice – Positive
Test for Saponin glycoside – Positive
TLC (Thin layer chromatography)
RF value of Standard was found to be- 0.45
RF value of Test was found to be- 0.41
UV-Spectrophotometer
UV maximum of Standard- 248 nm
UV maximum of Test – 230 nm
IR-Spectroscopy
 |
Figure 2(a): Showing IR Spectra of Standard sample.
|
 |
Figure 2(b): Showing IR Spectra of Test sample.
|
Table 1: Showing Interpretation of Standard and Test Glycyrrhizin sample
| S. No . | Peaks Standard | Sample |
| 1.
2. 3. 4. 5. 6. 7. |
-OH Stretching (Acid group )
C=O (Acid group) C-O (Acid group) C-H Stretching C=C group C=0 Ketone group OH Stretching |
2964 cm-1 2923 cm-1
1712 cm-1 1699 cm-1 1217 cm-1 1213 cm-1 2873 cm-1 2854 cm-1 1643 cm-1 1610 cm-1 1712 cm-1 1725 cm-1 3400-2400 cm-1 3398 cm-1 |
Behavioral Study
Effect on Hyperalgesia-
The daily treatment with Glycyrrhizin and Fluoxetine increases the tail withdrawal time of stressed animals significantly.
Table 2: Effect of Glycyrrhizin and Fluoxetine treatment on Hyperalgesia at 6th day
of chronic immobilization stress in mice.
| S.no. Groups Tail
|
Withdrawal Time (Sec)
Mean ±SEM |
| 1 Normal
2 Control (stressed) 3 Glycyrrhizin 4 Glycyrrhizin 5 Fluoxetine |
7.167±0.3651
5.000±0.3651 (100mg/kg) 10.67±0.4944*** (200mg/kg)11.50±0.4282*** (10mg/kg) 9.500±0.4282 |
Values are express in Mean±SEM. P<0.05, **Very Significant, ***Highly Significant as compare to Control group. (ANOVA followed by Dunnett’s test), n=5.
Table 3: Effect on Anxiety Daily treatment with Glycyrrhizin and Fluoxetine significantly decreased latency to enter, increased the no of entries and time spent in mirror chamber as compared to control (stressed) group.
| S.no. | Groups | Latency to
Enter(Sec) (Mean ±SEM) |
no.of Entries
(Mean±SEM) |
Time spent
(Sec) (Mean±SEM |
| 1 | Normal | 58.83±7.786 | 4.667±0.4216 | 31.33±3.528 |
| 2 | Control (stressed) | 110.0±6.952 | 2.000±0.3651 | 16.83±2.272 |
| 3 | Glycyrrhizin (100mg/kg) | 72.33±4.256*** | 5.500±0.5627** | 25.17±1.537* |
| 4 | Glycyrrhizin (200mg/kg) | 78.00±5.000** | 6.500±0.1000** | 37.50±2.500** |
| 5 | Fluoxetine (10mg/kg) | 80.00±5.323** | 4.833±0.3073** | 26.67±1.978* |
Values are express in Mean ±SEM. P < 0.05 *Significant, ** Very Significant ***Highly Significant as compare to Control group. (ANOVA followed by Dunnett’s test), n=5.
Effect on Locomotion
Daily treatment with Glycyrrhizin and Fluoxetine decreases locomotor activity when compared with control (stressed) group.
Table 4: Effect of Glycyrrhizin and Fluoxetine treatment on Locomotor activity at 6th
day of chronic immobilization stress in mice.
| S.no. | Groups | no. of counts/10min
Mean ±SEM |
| 1 | Normal | 386.8±37.54 |
| 2 | Control (stressed) | 560.5±59.39 |
| 3 | Glycyrrhizin (100mg/kg) | 394.5±47.61* |
| 4 | Glycyrrhizin (200mg/kg) | 270.5±25.50** |
| 5 | Fluoxetine (10mg/kg) | 233.0±11.13*** |
Values are express in Mean±SEM. P<0.05 *Significant, **Very Significant ***Highly
Significant as compare to Control group. (ANOVA followed by Dunnett’s test), n=5.
Effect on Muscle Co-ordination
The daily treatment with Glycyrrhizin and Fluoxetine increases the fall off time significantly when compared to control (stressed) group.
Table 5: Effect of Glycyrrhizin and Fluoxetine treatment on Muscle coordination at
6th day of chronic immobilization stress in mice
| S.no. | Groups | Fall off Time(sec.)
Mean ±SEM |
| 1 | Normal | 115.5±9.025 |
| 2 | Control (stressed) | 40.50±7.018 |
| 3 | Glycyrrhizin (100mg/kg) | 165.5±36.21** |
| 4 | Glycyrrhizin (200mg/kg) | 270.5±25.50*** |
| 5 | Fluoxetine (10mg/kg) | 114.2±4.167* |
Values are express in Mean±SEM. P<0.05 *Significant, **VerySignificant ***Highly
Significant as compare to Control group. (ANOVA followed by Dunnett’s test), n=5.
Effect on Memory
Daily treatment with Glycyrrhizin and Fluoxetine prevented the cognitive dysfunction significantly as compared to control (stressed) group.
Table 6: Effect of Glycyrrhizin and Fluoxetine treatment on Memory at 6th day of
chronic immobilization stress in mice
| S.no. | Groups | Latency to Enter (Sec)
Mean ±SEM |
| 1 | Normal | 5.525±1.404 |
| 2 | Control (stressed) | 10.72±0.6536 |
| 3 | Glycyrrhizin (100mg/kg) | 4.543±0.1299*** |
| 4 | Glycyrrhizin (200mg/kg) | 5.827±0.9467* |
| 5 | Fluoxetine (10mg/kg) | 4.627±0.3495** |
Values are express in Mean±SEM. P<0.05 *Significant, ** Very Significant ***Highly
Significant as compare to Control group. (ANOVA followed by Dunnett’s test), n=5.
Discussion
Stress is known to induce alterations in various physiological and psychological responses even leading to pathological diseases. The stress induced effects are supposed to be an outcome of altered activity of different mechanisms such as Central neurotransmitter, Neurohormonal factors, particularly those linked with the pituitaryadrenal axis and free radical generation. Exposure to stress caused significant behavior and biochemical changes. Chronic immobilization stress is the most widely used method for assessing the antistress property of a novel compound. In the present study, chronic immobilization stress caused impairment of muscle coordination, locomotion, anxiety, cognitive functions and hyperalgesia. Immobilization stress increases 2-3 fold of plasma corticosterone level due to activation of Hypothalamic-Pituitary-Adrenal axis (H-P-A axis) resulting in increased production of corticosterone. In humans and animals, adrenal cortex contains a higher concentration of ascorbic acid than other tissues and the acute administration of adrenocorticotropic hormone (ACTH) caused decrease in ascorbic acid levels. Increased cortisol level has been linked with anxiety like behavior and painful responses in humans. Stress may also cause oxidative stress and the formation of free radicals. Oxidative stress can cause cellular damage and neurodegeneration by inducing the reactive oxygen species (ROS) that oxidizes vital cellular components such as lipids, proteins and DNA. Stressed animals showed an early fall-off from the Rota-rod, increased anxiety response in mirror chamber, increased locomotor activity in actophotometer, hyperalgesic response and cognitive dysfunction with altered concentration and memory. Chronic immobilization stress also caused significant oxidative damage in animals brains indicated by increased lipid peroxidation, protein, nitrite activity and depleted reduced glutathione and catalase level in stressed brain. Daily treatment with Glycyrrhizin (100 & 200 mg/kg, p.o.) and Fluoxetine (10 mg/kg, i.p.) causes significantly increased the fall-off time, decreased latency to enter in mirror chamber, decreased locomotor activity, decreased the hyperalgesic responses and prevented the memory dysfunction. Glycyrrhizin also significantly decreased the level of lipid peroxidation, protein, nitrite and increased the activity of endogenous antioxidants such as reduced glutathione and catalase in the brain. Glycyrrhizin also reversed the decrease level of adrenal ascorbic acid in stressed animals. Antistress activity of Glycyrrhizin may be due to attenuating the H-P-A axis activation and free radical scavenging activity (Antioxidant activity). In summary, the present study revealed that daily treatment with Glycyrrhizin (100 & 200 mg/kg, p.o.) was effective in reversing chronic immobilization stress induced various behavioral and biochemical alteration in mice.
Conclusion
The current research concludes that Glycyrrhizin at the doses of 100 and 200 mg/kg, p.o. reversed the behavioral and biochemical changes in Chronic Immobilization Stressed mice. So we can predict that Glycyrrhizin, the active constituent of liquorice shows antistress potential.
References
- Stress management [Online]. 2004 [cited 2009 Sep 03]; Available from :URL:http://www.lifepositive.com/mind/psychology/stress/stress-management.asp Gupta V, Gupta A. Anti-stress and Adaptogenic activity of L- Arginine supplementation. Advance Access Publication 2004 Dec 4;2(1):93-97.
- Bhatwadekar AD, Chintawar SD. Antistress activity of Butea monosperma flowers. Indian Journal of Pharmacology 1999;31:153-155.
- Belmonte J. Signs of stress [Online]. [2008?] [cited 2009 Sep 03]; Available from:URL:http://www.helpguide.org/mental/stress-signs.htm
- Causes of stress [Online]. 2004 [cited 2009 Sep 03]; Available from: URL:http://www.lifepositive.com/mind/psychology/stress/causes-of- stress.sp
- Types of stress [Online]. [2001?] [cited 2009 Sep 03]; Available from: URL:http://changingminds.org/explanations/stress/stress-types.ht
- Tortora GJ, Grabowski SR. Principles Of Anatomy And Physiology. 8th ed. New York: Harper Collins College; 2004. P.542-545.
- Wales J, Snow M. Adaptogen [Online]. 2009 [cited 2009 Sep 24]; Available from: URL:http://en.wikipedia.org/wiki/Adaptogen
- Wales J, Snow M. Antioxidant [Online]. 2009 [cited 2009 Sep 24]; Available from: URL:http://en.wikipedia.org/wiki/Antioxidant
- Govindarajan R, Vijayakumar M, Pushpangadan P. Antioxidant approach to disease management and the role of rasayana herbs of Ayurveda. Journal of Ethnopharmacology 2005;99:165–178.
- Gupta D. The Herbs. 1st ed. India:Rajlaxmi Offset Printers;2008. P. 233-239.
- Liquroice. [Online]. 2009 [cited 2010 Feb 22]; Available from: URL:http://www.liquorice-wikipedia.mht.
- Kokate CK, Purohit AP. Textbook of Pharmacognosy. 3rd ed. India:Nirali Publication; P. 212-216.
- Indian Herbal Pharmacopoeia. New ed. India;2002. P. 243-253.
- Kiritikar KR, Basu BD, Inidian medicinal plant. 2nd ed. P. 727-728. (Vol I).
- Kazuo O, Yuko K, Lawrence L Susumu T. Glycyrrhizin inhibits prostaglandin E2 production by activated peritoneal macrophages from rats. Prostaglandins and Medicine 1981 Nov;7(5):457-46.
- Kenzo O, Nakao I. Inhibitory effect of glycyrrhizin on polypeptide phosporylation by polypeptide-dependent protein kinase (Kinase P) in vitro. Biochemical and Biophysical. Research Communications 1988 Dec 15;157(2):597-604.
- Masahiko I, Akihiko S, Kazuhiro H, Fuminori T. Mechanism of inhibitory effect of glycyrrhizin on replication of human immunodeficiency virus (HIV). Antiviral Research 1988 Dec 11;10(6): 289-298.
- Imanishi N, Kawai H, Hayashi Y, Yatsunami K, Ichikawa A. Effects of glycyrrhizin glycyrrhetinic acid on dexamethasone-induced changes in histamine synthesis of mouse mastocytoma P-815 cells and in histamine release from rat peritoneal mast cells. Biochemical Pharmacology 1989 August 1;38(15):2521-2526.
- Toshio H, Shojiro I, Atsushi K, Shuzo M, Yosuke M. Preliminary evidence for inhibitory effect of Glycyrrhizin on HIV replication in patients with AIDS. Antiviral Research 1989 Jun-Jul;11(5-6): 255-261.
- Ryoichi H, Tetsuhiro M, Keiko M, Sohei Y, Kenji T,Nobuo Y. Myotonic and repetitive discharges in hypokalemic myopathy associated with glycyrrhizin-induced hypochloremia. Journal of the Neurological Sciences 1992 Jan;107(1):74-77.
- Nakajima N, Utsunomiya T, Kobayashi M, Herndon DN, Pollard RB. In-vitro induction of anti-type 2 T cells by glycyrrhizin. Burns 1996 Dec;22(8):612-617.
- Ge L, Ivo PN, Tin-Yan C. The effects of pretreatment with glycyrrhizin and glycyrrhetinic acid on the retrorsine-induced hepatotoxicity in rats. Toxicon 1999 Sep;37(9):1259-1270.
- Monica M, Mario C, Andrea B, Claudio C,Tiziana R. Effect of glycyrrhizin and its diastereoisomers on the growth of human tumour cells: preliminary findings. Phytotherapy Research 1998 Dec 18;12(S1):S95-S97.
- Paolini M , Broccoli M, Perocco P, Cantelli-Forti G. Effect of liquorice and glycyrrhizin on rat liver carcinogen metabolizing enzymes. Cancer Letters 1999 Oct 18;145(1-2):35-42.
- Chao CH, Wen-Kang C, Pel-Hu L, Wei-Che Y. Synergistic effect of cadmium chloride and acetaldehyde on cytotoxicity and its prevention by quercetin and glycyrrhizin.2001 Sep 20;117-127.
- Hong DJ, Iwatani Y, Ishida T. Glycyrrhizin enhances interleukin-12 production in peritoneal macrophages. Immunology 2003;103:235±243.
- Tokuichiro U, Makiko K, Masahiko I, David NH, Richard BP. Glycyrrhizin restores the impaired IL-12 production in thermally injured mice. Cytokine 2001 Apr;14(1):49-55
- Jean MC, Natale S, Alain J, Daniel G. Interferon, ribavirin, 6-azauridine and glycyrrhizin: antiviral compounds active against pathogenic flaviviruses. Antiviral Research 2003 Mar;58(1):73-79.
- Wallace MS, Mariane A, Bruno R. Antithrombotic effect of Glycyrrhizin, a plantderived thrombin inhibitor. Thrombosis Research 2003;112(1-2):93-98.
- Sachiko M, Hiroatsu M, Yoshiko S, Kumi A. Glycyrrhizin and related compounds down-regulate production of inflammatory chemokines IL-8 and eotaxin 1 in a human lung fibroblast cell line. International Immunopharmacology 2004;4(13):1633-1644.
- Teruko I, Michinori S, Hiroshi O, Hidekazu A, Masaki O. Absorption-enhancing effect of glycyrrhizin induced in the presence of capric acid. International Journal of Pharmaceutics 2005 Apr 27;294(1-2):11-21.
- Linn CJ, Cherng JM, Hung MS. Inhibitory effects of some derivatives of glycyrrhizic acid against Epstein-Barr virus infection:Structure–activity relationships. Antiviral Research 2008;79:6–11.
- Masahide Y, Yuji M. Effects of glycyrrhizin on immune-mediated cytotoxicity. Journal of Gastroenterology and Hepatology 2008 Jun 18;12(3):243-248.
- Menegazzi M, Rosanna DP, Emanuela M. Glycyrrhizin attenuates the development of carageenan-induced lung injury in mice. Pharmacological Research 2008;58:22–31.
- Panneerselvam K, Kodukkur VP. Antihyperglycemic effect of 18P-glycyrrhetinic acid, aglycone of glycyrrhizin, on streptozotocin-diabetic rats. European Journal of Pharmacology 2009 Mar 15;606(1-3):269-273.
- Qiaogen Z, Ping W, Jing L. Simultaneous determination of 18- and 18-glycyrrhetic acid in human plasma by LC-ESI-MS and its application to pharmacokinetics. Biomedical Chromatography2008 Oct10;23(1):54-62.
- Tripathi M, Singh BK, Kakkar P. Glycyrrhizic acid modulates t-BHP induced apoptosis in primary rat hepatocytes . Food and chemical toxicology 2009;47:339- 347.
- Wolkerstorfer A, Kurz H, Bachhofner N, Szolar O. Glycyrrhizin inhibits influenza A virus uptake into the cell. Antiviral Research 2009;83:171–178.
- Xu-Ying W, Ming L, Dong LX, Ping H. Hepatoprotective and antihepatocarcinogenic effects of glycyrrhizin and matrine. Chemico-Biological Interactions 2009;181:15–19.
- Mcwatters A. Nutritional and herbal therapy for stress [Online]. 1997 [cited 2009 Sep Available from: URL:http://www.africangreys.com/articles/nutrition/nut-herbal.html
- Dhingra D, Parle M, Kulkarni SK. Memory enhancement of glycyrrhiza glabra. Journal of Ethanopharmacology 2004 Jan 15;361-365.
- Govindarajan R, Vijayakumar M. Antioxidant approach to disease management and the role of rasayana herbs of Ayurveda. Journal of Ethnopharmacology 2005;99:165-178.
- Kokate CK, Purohit AP. Textbook of Practical Pharmacognosy. 1st ed. India:Nirali Publication; P. 153.
- Kokate CK, Purohit AP. Textbook of Pharmacognosy. 3rd ed. India:Nirali Publicatio;215.
- Budavari S. The Merck Index. 12th ed. NJ: Merck Research Laboratories; 1996. p .4515.
- Pavia DL. Spectroscopy. 1st ed. India:Rajkamal Electric Press; p. 26-92.
- Das A, Kapoor K. Immobilization stress-induced change in brain acetylcholinesterase activity and cognitive function in mice. Pharmacological research 2000;42(3):213-217.
- Goyal R, Kumar A. Protective effect of alprazolam in acute immobilization stressinduced certain behavioral and biochemical alteration in mice. Pharmacological reports 2007;59:284-290.
- Kaur G, Kulkarni SK. Differential effect of a polyherbal formulation-OB-200G in male and female mice subjected to forced swim stress. Indian J Physiol Pharmacol 2000;44(3):281-289.
- Dhir A, Kulkarni SK. Venlafaxine reverse chronic fatigue-induced behavioral, biochemical and neurochemical alterations in mice.Wills ED. Mechanism of lipid peroxide formation in animal tissues. Journal of Biochemistry 1966;99:667-676.
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys 1959;82(48):670-677.
- Green LC, Wagner DA. Analysis of nitrate, nitrite and nitrate in biological fluids. Anal Biochem 1982;126:131-138.
- Lowry OH, Rosenvberg NJ. Protein measurement with the Folin-phenol reagent. J Biol Chem 1951;193:265-275.
- Luck H. Methods of Enzymatic Analysis. Academic Press 1971:885-893.
- Roe JH, Kuether CA. The determination of ascorbic acid in whole blood and urine through the 2,4-dinitrophenyl hydrazine derivative of dehydro ascorbic acid. J Biol Chem 1943;147:399-407.
- Indian Pharmacopoeia. 5th ed. Ghaziabad: The Indian Pharmacopoeia Commission; 2008. p. 503 (vol I).