Aliya Hayat¹, Rafia Azmat²* and Farha Aziz³
¹Department of Microbiology, 1,3Jinnah University for Women 5C Nazimabad - 746 00 Karachi (Pakistan). University of Karachi, Karachi - 757 20 (Pakistan).
²*Department of Chemistry, 1,3Jinnah University for Women 5C Nazimabad - 746 00 Karachi (Pakistan). University of Karachi, Karachi - 757 20 (Pakistan).
³Department of Biochemistry, 1,3Jinnah University for Women 5C Nazimabad - 746 00 Karachi (Pakistan). University of Karachi, Karachi - 757 20 (Pakistan).
Abstract
Pseudomonas stutzeri is an aerobic, green fluorescent bacterium which secretes green pigment in aerobic condition. In this article the release of bluish-green, luminous, water -soluble stain by P. stutzeri was discussed in presence of Cu metal in relation with the survival of P.stutzeri in nutrient medium. The activity of Pseudomonas stutzeri was monitored in provisions of colors of pigment with varying concentrations of Cu metal in petri dish. Results showed a variation in colors of pigment with different concentration of Cu metal. It was light green with water while colourless at high concentration of metal and blue with the moderate concentration of Cu. It was observed that high concentration of Cu results in the death of all microorganisms may be due to the reaction of Cu with oxygen or Cu responsible to decrease the BOD which ultimately affects the biosynthesis of pigment. No color of Cu with any life of Pseudomonas stutzeri at high dose also indicates that the Cu adsorbed on dead mass of bacterial strain. Less microbial life at low and moderate concentration of Cu with blue color reflects that Cu may be bonded with the pigment. It was concluded that Cu2+ was bactericidal to the phytopathogenic bacterium Pseudomonas stutzeri in the micromolar range which inhibits the biosynthesis of pigment at high concentration and bacteria experience death. It was suggested that pigmentation property of Pseudomonas stutzeri can be used as a monitoring tool of contaminated soil by heavy metals.
Keywords
Pseudomonas stutzeri; pigment; Cu
Download this article as:| Copy the following to cite this article: Aliya Hayat A, Azmat R, Aziz F. Effect of Cu on Pigmentation and Survival of Pseudomonas stutzeri. Biomed Pharmacol J 2012;5(1) |
| Copy the following to cite this URL: Aliya Hayat A, Azmat R, Aziz F. Effect of Cu on Pigmentation and Survival of Pseudomonas stutzeri. Biomed Pharmacol J 2012;5(1). Available from: http://biomedpharmajournal.org/?p=2221 |
Introduction
The production under assured intensified environment of yellow-green, fluorescent, water-soluble stain is a feature property of some Pseudomonas spp. (Stanier et al., 1966). They all belongs to the same intra-generic genetic homology group (Palleroni et al.,1973) and contain P. aeruginosa, P. putida, P. fluorescens and phytopathogens of the P. syringae type (Palleroni & Doudoroff, 1974).
Teitzel et al (2003) observed the effect of the heavy metals Cu, Pb, and Zn on bio-layer and planktonic Pseudomonas aeruginosa. The bio- layer and live culture were tested for heavy metal tolerance through rotating-disk biofilm reactor and found that biofilm was more resistant to heavy metal as compared to live microbial cell. A probable justification for this is that the extra cellular polymeric materials that encase a biofilm may be accountable for shielding the cells from heavy metal pressure by combining the heavy metals and hold up their distribution within the biofilm.
Meyer et al., (1978) reported that iron-deficiency was the major source in bioproduction of a yellow-green, fluorescent, water-soluble pigment by Pseudomonas fluorescens which was not directly affected by the environment of the organic carbon basis. They also determined the spectral properties of the pure pigment, its molecular weight (1500 ± 75) and its stability constant for Fe3+ (of the order of 1032) and suggest that the biosynthesis and its chemical properties (formation of a stable Fe3+ complex) showed that the fluorescent pigment was a desferrisiderophore.
Detailed literature survey on the fluorescent pigment synthesized by a strain of Pseudomonas sp. showed that it forms a stable complex with metals. But no report is available yet which describe the role of Pseudomonas stutzeri as a bio monitoring tool to explain the soil contamination through its color of pigmentation. This article describes pigment synthesis in relation with heavy metal Cu, its effect on bacteria, Cu removal and role in complex formation.
Material and methods
The strain of Pseudomonas stutzeri employed was isolated from sample taken from heavy metal contaminated soil. The organism was held in nutrient agar as Ps 01B. The biochemical characteristics of oxidase positive organism were identified by QTS-NE Strips (Desto laboratories) (Table 1). Nutrient agar and nutrient broth were used, for the experiments, contains no source of Cu or Fe.
Table 1: Identification Of Microorganism By Qts-Ne Kit
| Test | Reaction | Identified Specie | |
| IND | Indole | Negative | “Pseudomonas stutzeri” |
| GLU
(Acid) |
a)Acid from Glucose
b)Nitrate reduction |
Positive | |
| ADH | Arginine dihydrolase | Negative | |
| URE | Urea hydrolysis | Negative | |
| ESC | Esculin hydrolysis | Positive | |
| GEL | Gelatin hydrolysis | Positive | |
| ONPG | O, nitrophenyl β D galactosidase | Negative | |
| GLU | Glucose assimilation | Positive | |
| MAL | Maltose assimilation | Negative | |
| MNE | Mannose assimilation | Positive | |
| NAG | N-acetyl-glucose amine-assimilation | Negative | |
| CIT | Sodium citrate assimilation | Positive | |
| CO
Strip |
Spot test Cytochrome oxidase | Positive | |
Culture was maintained in 5 ml nutrient broth. 200ug/ml conc. of CuSO4 was made in nutrient broth. Two fold serial dilution of CuSO4 was made for the identification of survival and pigment production of Pseudomonas stutzeri. 0.1 ml culture was inoculated in each tube separately and incubated at 370 C.
Bacterial intensification was estimated turbidometrically at 600 mm, to conclude the concentration of fluorescent pigment in culture media. Microorganisms were removed via centrifugation and the optical density of supernatant liquid was measured at 400nm. Survival of bacteria in different concentration of CuSO4 was determined on nutrient agar by serial dilution method. Water and Hoagland solution in nutrient broth was taken as control.
Results and Discussion
This study specifies some observations regarding the stress of Cu on the intensification and pigmentation of bacteria, Pseudomonas stutzeri which were monitored on nutrient medium in presence of aqueous and Cu concentration. Activity was determined as a function of pigment secretion and survival of bacteria under metal stress (Meyer et al., 1978). Survival and optical density were represented in Figures (1-3). The change of color of pigmentation under the influence of copper on nutrient medium naturally suggested an affect of addition of the biologically important metal ions Cu2+, on the growth and the pigmentation of bacteria, as well as possible “detoxification” of the metals by bacteria. A probable mechanism of Cu detoxification or binding of Cu on dead biomass Pseudomonas stutzeri was discussed at high concentration. Influence of Cu concentration in serial dilution viz 200 to .39 µgm/ ml on growth and secretion of pigment gives some interesting results which were easily observe through the color of pigment of Pseudomonas stutzeri presented in Fig. 1.
 |
Figure 1: Pigment production by Pseudomonas at different concentration of CuSO4 |
Results suggest that growth of Pseudomonas stutzeri in the typical medium (which included no Cu) was escorted by release of pigment whereas excretion finished by adding different concentration of Cu. Highest concentration of Cu to the culture medium almost inhibit the growth yield with completely repressed formation of color of pigment when Cu was in the range of 50 – 12.5ug/mL concentration. The last four dilution (3.13 – 0.39ug/mL) showed an increase in pigment production, indicates that Cu in its negligible amount had no effect on microbial growth and pigment production (Table 2&3). The quantity of stain produced per unit of cell mass was contrary to the initial Cu concentration of the medium.
Table 2: Effect of Variable concentration of Cu on pigment Color
| DILUTION | COPPER CONCENTRATION
µg/ml |
PIGMENTATION |
| Tube 1 | 200µg/ml | No |
| Tube 2 | 100µg/ml | No |
| Tube 3 | 50 µg/ml | Light Green color |
| Tube 4 | 25µg/ml | Light Green color |
| Tube 5 | 12.5µg/ml | Light Green color |
| Tube 6 | 6.25µg/ml | Green color with blacking |
| Tube 7 | 3.13µg/ml | Green color |
| Tube 8 | 1.56µg/ml | Green color |
| Tube 9 | 0.78µg/ml | Green color |
| Tube 10 | 0.39µg/ml | Green color |
Table 3: Survival Of Pseudomonas In The Presence Of Different Concentration Of CuSo4
| TUBES | AVERAGE
NO. OF COLONIES |
LOG10 | O.D |
| 01 | No growth | No growth | 0.28 |
| 02 | No growth | No growth | 0.25 |
| 03 | 593 | 2.777 | 0.21 |
| 04 | 5370 | 3.729 | 0.24 |
| 05 | 20493 | 4.312 | 0.24 |
| 06 | 708 | 2.580 | 0.26 |
| 07 | 130 | 2.114 | 0.36 |
| 08 | 921 | 2.964 | 0.43 |
| 09 | 1946 | 3.299 | 0.40 |
| 10 | 108400 | 5.035 | 0.44 |
Exposure of Pseudomonas stutzeri cells to Cu2+ at high concentration alone resulted in quick and marked cell loss, and binding of nearly all of the copper in solution (Finazzi-Agro et al.1970). The results illustrated that, at an effective concentration, feeble and reasonable copper-ligands can efficiently upset copper toxicity. Experiment showed number of interesting facts. In the Fig.1growth of bacteria in the tubes, indicates a distinct difference in tolerance of the diverse concentration of Cu. Thus, when both low and high copper concentration in dilutions of 200 to 100 µgm/ ml, were added to agar, inhibit the growth of pseudomonas and pigmentation of interest (Muayad et al 2009). Low concentration of Cu added to the medias has no marked effect on pigmentation (Table 2 and Fig. 1). The addition of copper to the medium does not affect the growth at low concentration but effects on change of the color, (Fig. 1), although the shade was altered in some instances as reported in the Table (2). It may be related with growth of bacteria in Cu stress which showed varied results with the different concentration of Cu (Table 3). At higher concentration of Cu death of all bacteria were occurred which may be related with negative effects of Cu on bacteria growth or absorption of oxygen by Cu to form cupric oxide which act as a toxicant for bacteria but no color of Cu in the medium showed that Cu adsorbed on the dead mass of cell which was earlier investigated by Chang et al.(2007 & 1997) who reported that the Pseudomonas aeruginosa and Bacillus thuringiensis act as a good biosorbent for heavy metal removal in contaminated location specially for Hg and Cu. The burly interfaces of mercury and copper with organic stuff propose that these adverse elements might be eliminated from the surroundings by bacterial catching and appropriation (Hassen et al 1998). It is interested that metal in polluted environments existed in different forms. The growth phase exhibited no effects on the adsorption of Cu (Chang et al 1997). The loss of Cu color at high concentration was not due to the reduction of Cu by the pigment but may be due to the adsorption of Cu on dead mass of Pseudomonas stutzeri which indicated that the dead mass of Pseudomonas was effective to control the Cu toxicity as reported earlier that dead mass of the cells are effective in controlling the toxicity of heavy metal (Hassen et al 1998). Chen et al (2007) reported that the results of laboratory equilibration studies which also indicated that biomass-adsorbed Cu (II) or Zn(II) portions may be included both reversibly and powerfully bounded or resistant components.
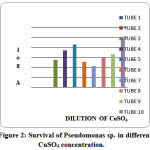 |
Figure 2: Survival of Pseudomonas sp. in different CuSO4 concentration |
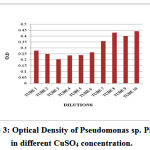 |
Figure 3: Optical Density of Pseudomonas sp. Pigment in different CuSO4 concentration. |
Some authors (Meyer et al 1978) have recognized an important role of nature of the organic carbon and energy source in pigment synthesis; showed influence on pigment production which are classified as ‘chromogenic’ or ‘anti-chromogenic’. It was observed that when Cu concentration decreases, the growth of Pseudomonas stutzeri increases and significant quantity of fluorescent pigment were produced (Table 1)when standard nutrient medium was pretreated with Hoagland nutrient medium to check the effect on production of pigment, an increase yield of Pseudomonas stutzeri was reduced with low pigment production or may be due to an ‘antichromogenic’ substrate can thus be converted into a ‘chromogenic’ one by a specific reduction of the Cu concentration of the medium (João P.S. Cabral 1994 and 1991). However, the precise Cu supplies of Pseudomonas stutzeri may vary as a function of the organic carbon and energy source.
Conclusion
The general conclusion of above report based on justification of the effect of different doses of Cu metal in general on pigmentation and survival. Naturally, there would be an optimum effective metal concentration on each organism and in this study it was above 50 µgm/ ml,
The addition of Cu, on the medium containing low concentration of copper produced a good intensification of pigment with change in color from light green to blue which clearly indicate the binding of Cu with the pigment but however at a concentration higher than 100 µgm/ ml, it turns colorless which indicate two possibilities 1) reduced state of pigment and 2) Cu binding with dead mass of strain or may be the fact that in the presence of metals some ordinarily pigmented bacteria grow without producing a pigment as in the case of Staphylococcus aureus, Pseudomonas pyocyanea, or to a different color as in the case of Chlorella (Kharasch et al 1936). This study also suggest that pigment secretion in presence of metal can also be used as a bio tool for detection of heavy metal concentration in soil through inoculation of bacteria in soil where survival of bacteria determine the toxicity level however more researches are required to take under consideration with different metal and different bacterial strain
References
- Chen X, Wu W, Shi J, Xu X, Wang H, Chen Y.2007 Adsorption of copper and zinc on Pseudomonas putida CZ1: particle concentration effect and adsorption reversibility. Colloids Surf B Biointerfaces. 15;54(1):46-52.
- Finazzi-Agro, A., Rotilio, G, Avigliano,L., Guerrieri,P Boffi, V., Mondovi, B.1970 Environment of copper in Pseudomonas fluorescens Azurin: fluorometric approach Biochemistry, 9 (9): 2009–2014
- Hassen A., Saidi, N., Cherif and Boudabous ,A. , 1998 Effects of heavy metals on Pseudomonas aeruginosa and Bacillus thuringiensis Bioresource Technology 65(1-2), 73-82
- Jo-Shu Chang, Robin Law and Chung-Cheng Chang (1997) Biosorption of lead, copper and cadmium by biomass of Pseudomonas aeruginosa PU21 .Water Research 31(7):1651-1658
- João P.S. Cabral 1991The antibacterial action of cupric ions in Pseudomonas syringae FEMS Microbiology Letters 79(2-3):303-308
- João P.S. Cabral 1994 Influence of organic ligands on the toxicity of copper to Pseudomonas syringae FEMS Microbiology Letters. 117,(3): 341-344
- Kharasch M. S., Conway E. A. and Bloom W. 1936 Some chemical factors influencing growth and pigmentation of certain microorganisms’ Journal of bacteriology, 32( 5)533-540.
- Gail M. Teitzel and Matthew R. Parsek., 2003 Heavy Metal Resistance of Biofilm and Planktonic Pseudomonas aeruginosa Applied and Environmental Microbiology,69(4):2313-2320
- Muayad M. Abboud, Humodi A. Saeed, Khaled A. Tarawneh, Khaled M. Khleifat and Amjad Al Tarawneh 2009 Copper Uptake by Pseudomonas aeruginosa Isolated from Infected Burn Patients Current Microbiology 59(3)282-287
- Meyer J. M. and Abdallah M. A. (1978), The Fluorescent Pigment of Pseudomonas fluorescens: Biosynthesis, Purification and Physicochemical Properties Journal of General Microbiology 107 319-328; DOI 10.1099/00221287-107-2-319
- Palleroni, N. J., Kunisawa, R., Contopoulou, R. & Doudoroff, M. (1973). Nucleic acid homologies in the genus International Journal of Systematic Bacteriology 23, 333-339.
- Palleroni, N. J. & Doudoroff, M. (1974). The genus In Bergey’s Manual of Determinative Bacteriology, 8th edn, pp. 217-243. Edited by R.E. Buchanan & N.E. Gibbons. Baltimore: Williams & Wilkins.
- Stanier, R. , Palleroni, N. J. & Doudoroff, M. (1966). The aerobic pseudomonas : a taxonomic study. Journal of General Microbiology 43, 159- 271.







