Muhammad Nor Omar¹, Nor Hazwani M. Hasali¹, Naik T. Khan², Syed Faraz Moin² and Helmi Y. Alfarra¹
1Department of Biotechnology, Kulliyyah of Science, International Islamic University Malaysia, (Malaysia). ²International Center for Chemical and Biological Sciences, University of Karachi, Karachi (Pakistan).
Abstract
In recent years, artemisinin (1) has remained an effective drug to treat malaria. Numerous approaches have been adapted to increase the efficacy of (1) against antibiotic resistant malarial parasite. Microbial biotransformation of (1) has been used recently to produce promising derivatives of (1) on a large scale with low costs. During the last decade several biotransformation studies on (1), by using microorganisms, have been reported. This literature review focuses on the most recent microbial transformation studies on artemisinin and its derivatives.
Keywords
Artemisinin; Malaria; Biotransformation; Microorganisms; Hydroxylation
Download this article as:| Copy the following to cite this article: Omar M. N, Hasali N. H. M, Khan N. T, Moin S. F, Alfarra H. Y. Biotechnological Transformation of Artemisinin: Toward an Effective Anti-Malaria Drug. Biomed Pharmacol J 2012;5(1) |
| Copy the following to cite this URL: Omar M. N, Hasali N. H. M, Khan N. T, Moin S. F, Alfarra H. Y. Biotechnological Transformation of Artemisinin: Toward an Effective Anti-Malaria Drug. Biomed Pharmacol J 2012;5(1). Available from: http://biomedpharmajournal.org/?p=2187 |
Introduction
Biotransformation involves the chemical transformation of substrates to desired products by using enzymes, either purified or crude, from microorganisms (bacteria and fungus), plants or animals [1,2]. Transformation of compounds by the application of whole cell microorganisms is often advantageous as compared to isolated enzymes [3]. These days, microbial transformation is considered to be the inexpensively and ecologically competitive technology for the biotechnological specialists in looking for new methods to produce pure useful chemicals, pharmaceutical, and agrochemical compounds [4]. Microbial transformation has been extensively used, to create new and useful metabolites of almost all classes of terpenes, as a substitute of chemical synthesis for preparation of pharmacologically active compounds [3].
The current review focuses on the microbial transformation of artemisnin (1) during the recent time. Scifinder research tool was used to screen the literature of this related subject, and to the best of our knowledge, this is the first review about the microbial biotransformation of (1).
Artemisinin versus malaria
Therapeutic importance
Malaria has been a universal health problem which kills millions of people every year out of more than 300 million cases in the world. About 20% of childhood deaths in tropics are claimed to be due to malaria [5-7]. In Africa, the disease has killed at least one child in every 30 seconds (WHO). Plasmodium falciparum species is the most common cause of cerebral malaria. The continuously developing resistance of the malaria parasite to the anti-malarial drugs and the absence of successful anti-malarial vaccine challenged the researcher for the development of efficient anti-malarial drugs to treat this infection. The World Health Organization (WHO) considers artemisinin (1) (figure 1) and its derivatives as part of the best approach for malaria in Africa and South/east Asia [6, 8-10]. At present (1) has become the most effective way to deal with resistant strains of P. falciparum chlotoguine and mefloquine with minor side effects [6,8]. Moreover, (1) also shows effectiveness against other parasites in addition to some viral infections. Furthermore, (1) can be used in treatment of hepatitis B plus some cancer cell lines such as human leukaemia, breast and colon cancer [5,8].
Source of Artemisinin
Artemisinin, also known as qinghaosu, was isolated, in 1970s, from glandular trichomes of the herbal plant Artemisia annua Linn by Chinese researchers, and was identified as a sesquiterpene lactone endoperoxide. Artemisinin was found only in A. annua with amount of 0.01% to 1.4% in some cultivated strains. Recently (1) has been reported in other Artemisia species which are A. bushriences and A. dracuuculus var dracunculus with different traces [10,11,5,7]. A. annua has become an attractive supply of essential oils, while its commercial significance comes from the probabiltiy of being the only source of genus Artemisia to synthesize (1) [11]. Although, cost of (1) may be one of important restrictions for users of anti-malaria, it has been comprehensively reviewed that there are certain natural problems with current artemisnins that require discussion. It was reviewed that (1) based drugs (e.g., artemether and arteether) have neurotoxic and cardiotoxic effects in experimental animals, however, millions of doses in various formulations have been given to humans without significant evidence of major toxicity [12,13]. Artemisnin is relatively easily purified by crystallisation after extraction from A. annua plants but is extremely difficult to synthesise de novo [14,15]. The disadvantage of (1) is that it cannot be administrated orally or by rectal route. It can only be given via internal route because it is a highly crystalline compound that does not dissolve in oil or water. Semisynthetic derivatives of (1) i.e Artesunate, Artemether, Arteether, Dihydroartemisinin, artesunic acid and Artelinic acid have been chemically modified at the C10 position to overcome that problem [13,12].
Mode of action
Artemisinin and its analogues induce a very rapid reduction of blood parasites, starting almost directly after administration. The antimalarial action of (1) and its derivatives has been accredited to their chemical ability to produce free radicals. This approach of action has been suggested partially on the argument that, well standard sources of free radicals (such as tert-butylperoxide) can eradicate malaria parasites. Free radicals can be generated from peroxides in the presence of Fe2+ as catalyst. Carbon centred free radicals have been placed forward as principal intermediates in the process of killing the parasite, another mechanism for artemisinins, based on reserve of the malarial parasite’s calcium ATPase (sarcoplasmic endoplasmic reticulum calcium ATPase, SERCA) has been suggested [12,13]. Artimisisin has been shown to be involved in alkylation of proteins containing heme group like heamoglobin, catalase and cytocrome c [16]. The action of (1) at least in part is reported to be conducted by interference in heamoglobin catabolism inside the parasite. Artimisinin inhibit the heme polymerization by histidine rich protein II of Plasmodium falciparum. The hemozoins (malarial pigment) produced by the mature parasite were also broken down by (1) [17]. It has been found that monoalkylated and dialkylated haem derivatives produced by (1) can bind to histidine rich protein II and inhibit haemozoin formation. On the other hand generation and accumulation of these alkylated haemoglobin can bring about the death of parasite [18].
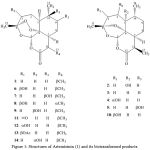 |
Figure 1: Structures of Artemisinin (1) and its biotransformed products |
Microbial transformation of Artemisinin
The scaling up production of artemisinin (1) from A.annua L. becomes the biggest challenge faced by researchers who are competing to find a potent drug against multi-drug resistant strain of malaria. In recent years, the chemical synthesis of artemisinin is not promising that much thus, biotransformation has been used as an option to produce it commercially and in large scales through modern biotechnology process. Sensitive nature, high recur rate, oil and water insolubility of (1) are disadvantages which limits its transformation by using extensive chemicals to develop a strong anti-malarial derivatives. Isomeric, rearranged, hydrolyzed and reduced products are all promising results of microbial transformation of (1) and its analogues. Furthermore, microbial transformations can be used to study mammalian metabolic pathways as it is reported in some successful studies which predicting the drug metabolism in mammals. Processes that can be involved in the biotransformation reactions of (1) include hydration reactions and breakdown of heterocyclic rings, hydroxylation of methyl, methyne and methylene group deoxidation reactions [7, 19, 20].
Aspergillus niger, the most common species of the genus Aspergillus which causes black mold disease on fruits and vegetables, transformed Artemisinin (1) into 1α-hydroxydeoxyartemisinin (2), deoxyartemisinin (3), 3α-hydroxydeoxyartemisinin (4) with yeild 15% , 2β-hydroxyartemisinin (5) with 80% yeild and with 19% yeild of 9β-hydroxyartemisinin (6) [19,21,22]. 18 et al., 2002a found 3 in the substrate controls without any microorganisms, which indicated that 3 was not a biotransformed product but a product of chemical reaction catalyzed by Fe2+ in the potato medium. A. flavus was also reported to transform 1 to 3 [20]. A. alliaceus, A. flavipes and A. parasiticus were also screened for the metabolism of 1, but there were no metabolites recorded from these species.
Cunninghaamella species have been used to metabolize a diverse range of drugs in a manner similar to that in mammals; these are thus used as microbial models for studying mammalian drug metabolism. Moreover, these fungi are also attractive to the researchers due to their ability to transform bioactive compounds. Contrary to Aspergillus, Cunninghaamella echinulata transformed 1 into a novel metabolite 10β-hydroxyartemisinin (7) with 50% yeild [19]. Cunninghamella elegans, on the other hand, transformed 1 into 9β-hydroxy-11α-artemisinin (8) as a minor metabolite with yeild 6% along with 4, 6 with 5.4%, 6.5% correspondingly and 7 which cosidered as the major metabolite with yeild of 78.6% [23]. Interestingly C. elegans gave 8 which was characterized by the inversion of stereochemistry at C-11. Cunninghaamella blakesleena was also screened for its ability to transform (1), but no transformation was obtained [24].
As a final point, more than 40 species of microorganisms have been reported to transform Artemisinin; only 11 of them have been used for complete transformation while 29 have been used only for preliminary screening. Mucor polymorphous reported to transform (1) to (6), 3β-hydroxyartemisinin (9), (3) and 3β-hydroxydeoxyartemisin (10) [21], while Williamson et al., 2007 reported three different culture collections of Mucor [25]. rammanianus to transform (1) to (4), (6), (7) and (8) with a relative variety of yields according to the variation of the culture collection type, in addition to that Mucor mucedo was used to transform the same substrate but there was no metabolites recorded [24].
Although that there are five different species of Streptomyces have been used to transform (1), only one species –Streptomyces griseus– has been reported to provide four different metabolites they were (4) with 9.5% , (6) with 16.1%, Artemisitone-9 (11) with 12.5% and 9α-hydroxyartemisinin (12) with 16.5% [25]. Lately Goswami et al., 2010 have reported two different compound that have not been reported from the transformation of (1) by using Penicillium simplissmum these two compounds described as 3β-acetoxyartemisinin (13) with 20.6% and 3α-hydroxyartemisinin (14) with 31.3%, as well in 1989 anther species of Penicillium which is Penicillium chrysogenum has been used by Lee et al,1989. and they reported for the first time the compounds (3) and (4). Eruotium amstelodami transformed (1) to (5) and (6) with yields 63% and 32% respectively [22], and the only Nocardia coralline species of this genus was used by Lee et al., 1989 to give (3).
Furthermore, many studies report the coversion of Artemisinin derivatives by microorganisms icluding conversion of 10- deoxyartemisinin to 5β-hydroxy-10-deoxyartemisinin, 4α-hydroxy-1,10-deoxoartemisinin , and 7β-hydroxy-10-deoxoartemisinin by C. elegans [27]; hydroxylation of 10-deoxoartemisinin to 15-hydroxy-10-deoxoartemisinin by Aspergillus niger [28]; 10- deoxyartemisinin to 7β-hydroxy-10 deoxyartemisinin by M. rammanianus [29]; conversion of artemisitene to 7β- hydroxy-9-epi-artemisinin by Aspergillus niger [30]; conversion of artemether to 7β-hydroxyartemether by Streptomyces lavendulae [31]; conversion of arteether to 7β-hydroxyarteether by C. elegans [32] and Beauveria sulfurescens [33].
Antimalarial activities of more than 14 trasformed metabolites of artemisinin and its derivatives that have been reported in this literature were evaluated, however, none of these metabolites have found superior in antimalarial activity to that of the original substrate.
Concluding remarks
To date, artemisinin has been obtained commercially from the cultivation of A. annua which makes it costly. As a result, there is a great need for better financial system pop up to produce artemisinin based drugs. Undoubtedly, any upgrading by conventional breeding is likely to lower the cost of artemisinin production. Depending on regulatory and related costs, biotransformation of artemisinin is still a promising opportunity that may help to produce novel derivatives. It is also much cheerful to observe the commercial significance in this area in the form of Dafra Pharma International. It was reported that microbial-derived (1) combination therapies might reduce the cost of the therapy from 30%-60% which might give a reasonable prices for artemisinin base combination therapy (ACT).
Artemisinin and its derivatives can be safe and well-tolerated antimalarial drugs. However, they can be inadequately to treat malaria as monotherapy, hence, moments should be invested in discovering more effective derivatives of (1) to a part of combination therapy for multidrug resistant malaria. On our ongoing research we are screening microorganisms that have not been used before as in the hope to find novel microoganisms that might produce novel bioactive metabolites of artemisinin.
Acknowledgement:
We would like to thank International Islamic University who is supporting our research and Mr. Omar Alqatami who supported us with some of the published materials.
References
- Muffler, K., Leipold, D., Scheller, M. C., Haas,C., Steingroewer, J., Bley, T., Neuhaus, H. E., Mirata,M. A., Schrader, J., Ulber, R “Biotransformation of triterpenes”, Process Biochemistry 46, 1-15 (2011).
- Baiping, M., Bing, F., Hongzhi, H., Yuwen C. “Biotransformation of Chinese Herbs and Their Ingredients”, Mode Tradit Chin Med Mater Med 12, 150-154 (2010 ).
- Demyttenaere, J. C. R. “Biotransformation of terpenoids by microorganisms”, Studies in Natural Products Chemistry 25, 125–178 (2001).
- Patel, R. N. “Synthesis of chiral pharmaceutical intermediates by biocatalysis”, Coordination Chemistry Reviews 252, 659-701 (2008).
- Mannan, A., Ahmad, I., Arshad, W., Asim, F. M., Qureshi, A. R., Hussain, I., and Mirza, B. “Survey of artemisinin production by diverse Artemisia species in northern Pakistan”, Malaria Journal, 299-310 (2010).
- Dhingra, V., Rao, K. V., Narasu, M. L. “Current status of artemisinin and its derivatives as antimalarial drugs”, Life Sciences 4, 279-300 (2000).
- Brown, G. D. “The Biosynthesis of Artemisinin (Qinghaosu) and the Phytochemistry of Artemisia annua L. (Qinghao)”, Molecules 15, 7603-7698 (2010).
- Hussain, I., Khan, F. U., Khan, L., Ayaz, S., and Khan, U. I. “Analysis of artemisinin in Artemesia species using high performance liquid chromatography”, world applied sciences journal 6, 632-636 (2010).
- Patel, S., Gaur, R., Verma, P., Bhakuni, R. S., and Mathur, A. “Biotransformation of artemisinin using cell suspension cultures of Catharanthus roseus (L.) G.Don and Lavandula officinalis L.”, Biotechnol Lett 32, 1167-1171 (2010).
- Liu, B., Wang, H., Du, Z., Li, G., and Ye, H. “Metabolic engineering of artemisinin biosynthesis in Artemisia annua L.”, Plant Cell Rep 30, 689-694 (2011).
- Goswami, A., Saikia, P. P. , Barua, N. C., Bordoloi, M.,Yadav, A.,Bora, T. C., Gogoi, B. K., Saxena, A. K., Suri, N., Sharma, M. “Bio-transformation of artemisinin using soil microbe: Direct C-acetoxylation of artemisinin at C-9 by Penicillium simplissimum”, Bioorganic & Medicinal Chemistry Letters 20, 359-361 (2010).
- Balint, G. A. “Artemisinin and its derivatives An important new class of antimalarial agents”, Pharmacology & Therapeutics 90, 261- 265 (2001).
- Woodrow, C. J., Haynes, R. K., Krishna, S. “Artemisinins”, Postgrad Med J 81, 71-78 (2005).
- China Cooperative Research Group “On qinghaosu and its derivatives as anti-malarials”. Trad Chin Med 2, 3–8 (1982).
- Bray P. G., Ward, S. A., O’Neill, P. M. “Quinolines and Artemisinin: Chemistry”, Biology and History, CTMI, 3-38 (2005).
- Ying, Ying-zi., Little, B., Meshnick, S. R., “Alkylation of proteins by artemisinin effect of heme, pH and drug structure”, Biochemical Pharmacology 48, 569-573 (1994).
- Pandey, A. V., Tekwani, B. L., Singh, R. L., Chauhan, V. S., “Artemisinin, an endoperoxide antimalarial, disrupts the hemoglobin catabolism and heme detoxification systems in malarial parasite”, The Journal of Biological Chemistry 274, 19383-19388 (1999).
- Kannan, R., Kumar, K., Sahal, D., Kukreti, S., Chauhan, V. S., “Reaction of artimesinin with haemoglobin: implications for antimalarial activity”, Biochemical Journal 385, 409-418 (2005).
- Zhan, J., Guo, H., Dai, J., Zhangb, Y., Guoa, “Microbial transformations of artemisinin by Cunninghamella echinulata and Aspergillus niger”, Tetrahedron Letters 43, 4519-4521 (2002).
- Srivastava, S., Luqman, S., Fatima, A., Darokar, M. P., Negi, A. S., Kumar, J. K., Shanker, K., Chanotiya, S. C., Sudeep, T., KHANUJA, S. P. S. “Biotransformation of Artemisinin Mediated through Fungal Strains for Obtaining Derivatives with Novel Activities”, Sci Pharm 77, 87-95 (2009).
- Zhan, J. X., Zhang, Y. X., Guo, H. Z., Han, J., Ning, L. L., Guo, D. A. “Microbial Metabolism of Artemisinin by Mucor polymorphosporus and Aspergillus niger”, J. Nat. Prod. 65, 1693-1695 (2002).
- Parshikov, I. A., Miriyala, B., Muraleedharan, K. M., Avery, M. A., Williamson, J. S. “Microbial transformation of artemisinin to 5-hydroxyartemisinin by Eurotium amstelodami and Aspergillus niger”, J Ind Microbiol Biotechnol 33, 349-352 (2006).
- Parshikov, I. A., Muraleedharan, K. M., Avery, M. A., Williamson, J. S. “Transformation of artemisinin by Cunninghamella elegans”, Appl Microbiol Biotechnol 64, 782-786 (2004).
- Lee, I. S., Elsholy, H. N., Croom, E. M., Hufford, C. D. “Microbial Metabolism Studies of the Antimalarial Sesquiterpene Artemisinin”, J Natural Products 52, 337-341 (1989).
- Williamson, S.J., Parshikov, A.I., Avery, A.M. “Microbial transformations of artemisinin” Editors. Singh, V. K.; Govil, J. N.; Arunachalam, C. Recent Progress in Medicinal Plants. 17; p 115-138 (2007).
- Liu, J. H., Chen, Y. G., Yu, B. Y., Chen, Y. J. “A novel ketone derivative of artemisinin biotransformed by Streptomyces griseus ATCC 13273”, Bioorganic & Medicinal Chemistry Letters 16 1909-1912 (2006).
- Parshikov, I. A., Muraleedharan, K. M., Miriyala, B., Avery, M. A., Williamson, J. S. “Hydroxylation of 10-Deoxoartemisinin by Cunninghamella elegans”, J. Nat. Prod. 67, 1595-1597 (2004).
- Parshikov I. A., M., B., Avery, M. A., Williamson, J. S. “Hydroxylation of 10-deoxoartemisinin to 15-hydroxy-10-deoxoartemisinin by Aspergillus niger”, Biotechnology Letters 26, 607-610 (2004).
- Fiaux de Medeiros, S., Avery,M. A., Avery, B., Leite, S. G.F., Freitas, A. C. C., Williamson J. S. “Biotransformation of 10-deoxoartemisinin to its 7β-hydroxy derivative by Mucor ramannianus”, Biotechnol Lett 24, 937-941 (2002).
- Orabi, K. Y., Galal, A. M., Ibrahim, A. S., El-Feraly, F. S., Khalifa, S. I., El-Sohly, H. N. “Microbial metabolism of artemisitene”, Phytochemistry 51 257-261 (1999 ).
- Abourashed, E. A., Hufford, C. D ”Microbial Transformation of Artemether”, J. Nat. Prod 59, 251-253 (1996).
- Hufford C. D., K. S. I., Orabi K. Y., Wiggers F.T. “1α-Hydroxyarteether, a new microbial transformation product”, J Nat Prod 58, 751-755 (1995).
- Ziffer, H., Hu, Y., Pu, Y. “Beauveria sulfurescens mediated oxidation of dihydroartemisinin derivatives”, NATO ASI Ser C Math Phys Sci 381, 361-373 (1992).







