K.Ravishankar* and N. Indira
Sri Sai Aditya Institute of Pharmaceutical Sciences and Research, Adb Road, Surampalem- 533 437 , East Godavari District (India).
Abstract
The present study deals with an antioxidant potential of Ethanolic leaf extract of Ricinus communis. Ricinus communis commonly known as castor oil belongs to the family Euphorbiaceae. In the present study antioxidant potential of Ethanolic leaf extract of Ricinus communis was investigated employing two different established in vitro methods , such as free radical scavenging activity using 1,1-diphenyl-2-picrylhydrazil (DPPH) and Reducing power assay. The results of the assay were then compared with reference standard Gallic acid. The Ethanolic extract of the bark of Ricinus Communis is a good source of compounds with antioxidant properties and the extract exhibited significant Free Radical Scavenging activity, Reducing power activity . Results obtained in the present study reveal ethanolic extract of Ricinus communis leaves posses significant antioxidant activity
Keywords
Ricinus communis; DPPH; Reducing power assay; Antioxidant activity
Download this article as:| Copy the following to cite this article: Ravishankar K, Indira N. Antioxidant Activity of Ethanolic Extract of Ricinus communis Leaf. Biomed Pharmacol J 2012;5(1) |
| Copy the following to cite this URL: Ravishankar K, Indira N. Antioxidant Activity of Ethanolic Extract of Ricinus communis Leaf. Biomed Pharmacol J 2012;5(1). Available from: http://biomedpharmajournal.org/?p=2436 |
Introduction
During last decade considerable attention has been focused on the involvement of Reactive Oxygen Species (ROS) in various diseases.Genaration of free radicals causes cumulative damage of DNA, proteins, lipids led to oxidative stress. This oxidative stress has been suggested to be the cause of ageing and various human diseases like cancer, hepatic disorders, and diabetes [1].DNA damage mediated by free radicals may result in mutation or chromosomal aberrations leading to carcinogenesis [2].Current research into free radicals has confirmed that food rich in antioxidants play an essential role in prevention of cardiovascular diseases and cancer [3]. Ricinus communis is an annual, perennial bush found throughout india, which is widely cultivated in the tropics & warm regions for castor oil production. In Indian system of medicine the leaf, root and seed oil of this plant have been used for the treatment of inflammation, hepatoprotective, hypoglycemic, laxative, diuretic, antibacterial, insecticidal, contraceptive, antifertility activity[4].The various phytochemical constituents like flavonoids (kaempferol-3-O-beta-d-rutinoside and kaempferol-3-O-beta-d-xylopyranoid) [5],phenolic compounds present in the plant extract may pocesses antioxidant property. Hence the free radical scavenging activity of the ethanolic extract of Ricinus communis leaf was evaluated using DPPH (2,2-diphenyl-1-picrylhydrazyl) and reducing power methods. The purpose of the present study was to evaluate the in vitro antioxidant activity of the ethanolic leaf extract of Ricinus communis.
Materials and Methods
Plant Collection
The leaves of the Ricinus communis were collected from the surrounding villages of Kakinada and the plant was identified and authenticated by the Botanist, Dr.T.U.Raghuram.
Preparation Of Extract
The freshly collected leaves of this plant were cleared from dirt then, dried under shade for about 15days and then coarsely powdered in a mechanical grinder. The powder was macerated with ethanol for 5 days, filtrate was collected & concentrated. The concentrated product was dried using desiccators with anhydrous calcium chloride. The percentage yield of the extract was 4%w/w.
Chemicals and Instruments
All the chemicals like Gallic acid (gifted from Glanxo Smith Kline), DPPH (purchased from Research-lab fine Chem.. Industries, Mumbai), Methanol, phosphate buffer, potassium ferricyanide, tricloroacetic acid, ferric chloride were used for this study are of analytical grade. UV-visible double beam spectrophotometer (Elico SL 210), Centrifuge machine, pH meter, Shimadzu electronic balance were used for analysis.
Antioxidant Activity
Free Radical Scavenging Activity
The free radical scavenging activity was followed by preparing 0.002% DPPH solution in methanol. Gallic acid was taken as the reference standard. Different concentrations of the extract (100, 200 and 500 µg/ml) and standard drug (1 and 2.5 µg/ml) were prepared using methanol. 1ml of 0.002% DPPH solution is mixed with 1ml of all the concentrations of both extract and standard separately. These mixtures are kept in dark for about 30min and the optical density was measured at 517nm. 0.002% DPPH and methanol mixture is the blank. Finally the % inhibition of the DPPH activity is calculated using the formula.
DPPH Scavenged (%) = [ (Acontrol– Atest ) / Acontrol ] × 100
Where Acontrol is the absorbance of the control reaction and Atest is the absorbance in the presence of the sample of the extracts. The antioxidant activity of the Ethanolic leaf extract was expressed as IC50 and compared with standard. The IC50 value was defined as the concentration (in μg/ml) of extracts that scavenges the DPPH radicals by 50%.
Reducing Power Method
Different concentrations of the extract (100, 200 and 500 µg/ml) and standard drug (1 and 2.5 µg/ml) were prepared using distilled water.1% potassium ferricyanide, 10% trichloro acetic acid, 0.1% ferric chloride and 0.2M phosphate buffer were prepared using distilled water. Gallic acid was taken as the reference standard. Then 1ml of each concentration of both extract and standard were taken separately and mixed with 1ml of 0.2M phosphate buffer (pH 6.6) and 1ml of potassium ferricyanide. Incubate all these samples at 500C for 20min. Then add 1ml of 10% trichloro acetic acid and centrifuge at 2000rpm for 10min. Now separate the upper layer (2.5ml) and then add (2.5ml) distilled water, 0.5ml of freshly prepared ferric chloride. Finally measure the absorbance at 700nm.
Results and Discussion
DPPH method
The DPPH radical scavenging assay is an easy rapid and sensitive method for the antioxidant screening of plant extracts[8] & the assay is known to give reliable information concerning the antioxidant ability of the tested compounds.A freshly prepared DPPH solution exhibits a deep blue color in the medium of methanol.Antioxidant molecules can quench DPPH free radicals (i.e.,by providing hydrogen atoms or by electron donation,via a free radical attack on the DPPH molecule) & convert them to colorless ( i.e.,2,2-diphenyl-1-hydrazine, or a substituted analogous hydrazine),result in a decrease in absorbance at 517nm.Hence the more rapidly the absorbance decreases, the more potent the antioxidant activity of the extract[9] .Table1 shows the % of DPPH radical scavenged by Gallic acid & Ethanolic leaf extract at various concentrations(µg/ml). Decrease in the concentration of DPPH radical due to the scavenging ability of the soluble constituents in the ethanolic extract of leaves of Ricinus communis and the standard Gallic acid, as a reference compound were observed (Figure-1a & 1b ) . The IC50 values were found to be 37.32μg/ml and 0.81 μg/ml for Ethanolic leaf extract of of Ricinus communis and Gallic acid respectively .
Reducing power assay
Measurement of reducing power gives antioxidant activity in the extract. In this assay, the yellow colour of the test solution changes to various shades of green and blue depending on the reducing power of each compound. Presence of reducers causes the conversion of the Fe3+/ferri cyanide complex used in this method to the ferrous form. By measuring the formation of Pearl’s Prussian blue at 700nm, it is possible to determine the concentration of Fe2+ ion[9]. The reducing power of the extract increased with increase in concentration,of Ethanolic leaf extract ( Table 2 , Figure-2 ) .
Table 1: Results of DPPH radical scavenging activity of ethanolic leaf extract of Ricinus communis
| Tested material | Concentration | % | IC50 (µg/ml) |
| (µg/ml) | Inhibition±SEM | ||
| Ethanolic leaf extract of Ricinus communis | 100
200 500 |
60.67±0.01
63.54±0.023 79.08±0.002 |
37.32 |
| Gallic acid | 1
2.5 |
56.19±0.0125
93.4±0.0007 |
0.81 |
Values are expressed as mean ± SEM
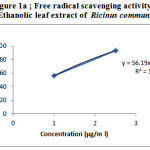 |
Figure 1a: Free radical scavenging activity of Ethanolic leaf extract of Ricinus communis |
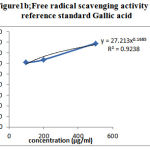 |
Figure 1b: Free radical scavenging activity of reference standard Gallic acid |
Table 2 : Effect of ethanolic leaf extract of Ricinuscommunis on Reducing power assay
| Tested material | Concentration(µg/ml) | Absorbance ±SEM |
| Ethanolic leaf extract of Ricinus communis | 100
200 500 |
0.295±0.026
0.491±0.020 0.947±0.009 |
| Gallic acid | 1
2.5 |
0.471±0.011
0.765±0.013 |
Values are expressed as mean ± SEM
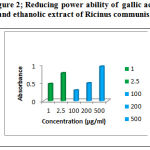 |
Figure 2: Reducing power ability of gallic acid and ethanolic extract of Ricinus communis. |
Conclusion
This study indicates the concentration and time dependent reducing ability of the Ethanolic leaf extract of Ricinus communis. The reducing ability of the extract of the plant can be ascribed to the presence of secondary metabolites in the plant. Further investigations may be carried out to isolate the compounds responsible for the activity and to find out the possible synergism existing between the compounds in the plant.
Acknowledgement
The authors are thankful to the Vice Chairman Sri.N.Satish Reddy , for providing necessary facilities and constant encouragement through out the research work carried out in the Institution.
References
- Tiwari AK, Anti-oxidants: New generation therapeutic base for treatment of polygenic disorders, Current Science, 86, 2004, 1092-1102.
- Athar M, Oxidative stress and experimental carcinogenesis, Indian J. Exp.Biology, 40, 2002, 656-657.
- Kris-Etherton PM, Hecker KD, Bonanome A, Coval SM, Binkoski AE, Hilpert KF,Bio active compounds in foods: their role in the prevention of cardio vascular disease and cancer, American Journal of Medicine, 113,1993, 71-88.
- Pradeep Pratap Singh., Ambika, Chauhan, S.M.S. Activity guided isolation of antioxidants from the leaves of Ricinus communis L. Food Chemistry, 114 (2009).
- Raju Ilavarasan., Moni Mallika, Subramanian Venkataraman. Anti-inflammatory and free radical scavenging activity of Ricinus communis root extract .Journal of Ethnopharmacology , 103, 478–480 (2010).
- Nayan Bhalodia, R., Pankaj Nariya,B. Acharya,R.N. and ShuklaV.J. Evaluation of in vitro Antioxidant activity of Flowers of Cassia fistula Linn. International Journal of PharmTech Research, 3, 589-599 (2011).
- Sonia Miladi., and Mohamed Damak.(2008).Invitro antioxidant activities of Aloe vera skin extracts. Journal de la Société Chimique de Tunisie,10, 101-109.
- Hemalatha,S., Lalitha,P. and Arulpriya,P.Antioxidant activities of the extracts of the aerial roots of Pothos aurea (Linden ex Andre), Der Pharma Chemica, 2(6): 84-86 (2010).
- Jayanthi,P.,and Lalitha,P. Reducing power of the solvent extracts of Echornia crassipes (mart) solms.International Journal of Pharmacy and Pharmaceutical Sciences 3,126-128 (2011).







