Manuscript accepted on :June 01, 2011
Published online on: 26-11-2015
Plagiarism Check: Yes
Raj Narayan Sharma¹, K. P. Sharma² and S. N. Dikshit²
¹Hindustan Institute of Technology Science and Management (R.G.P.V. University) India.
²SMS Govt. Model Science College, (Jiwaji university) Gwalior - 474 002 India.
Corresponding Aurhor E-mail: rajnarayan1974@gmail.com
Abstract
A series of bis-amide and hydrazide-containing derivatives of malonic acid and thiophenoladducts of acid hydrazones have been synthesized by the reaction of 2-[(N-acetyl) 2, 5-dichloroanilido] acetohydrazide with various Carbonyl Compounds in 44 to 69% yield. Newly prepared compounds have been tested for their anti-bacterial activity against gram positive bacteria S.albus, S.aureus and gram negative bacteria E.coli and Pseudomonas piosineus. The compound (1, 5, 15, 16) shown significant activities and compound (2, 4, 9, 12, 14) have shown moderate activity. The same compounds were tested for their anti-fungal activity against Candida albicans, Aspergillus niger and Alternaria alternata at concentration of 30 mg/ml using Savored dextrose agar media. The compound (3, 8, 11, 13) shown significant activities and compound (1, 7, 10, 17) have shown moderate activity against Candida albicans and Aspergillus niger. All the other compounds did not show significant activity against the fungi at the concentration used.
Keywords
Malonicacid; bis-amides; acidhydrazides; hydrazone-thiophenoladducts
Download this article as:| Copy the following to cite this article: Sharma R. N, Sharma K. P, Dikshit S. N. Synthesis of Bis-Amide and Hydrazide Containing Derivatives of Malonic Acid and Thiophenoladducts of Acidhydrazones Derived from 2-[(N-acetyl) 2, 5-dichloroanilido] Acetohydrazide. Biomed Pharmacol J 2011;4(1) |
| Copy the following to cite this URL: Sharma R. N, Sharma K. P, Dikshit S. N. Synthesis of Bis-Amide and Hydrazide Containing Derivatives of Malonic Acid and Thiophenoladducts of Acidhydrazones Derived from 2-[(N-acetyl) 2, 5-dichloroanilido] Acetohydrazide. Biomed Pharmacol J 2011;4(1). Available from: http://biomedpharmajournal.org/?p=1719 |
Introduction
Acidhydrazides and their condensation products possessing an azometine -NHN=CH- Proton constitute an important class of compounds for new drug development. In the past several years, numerous compounds with diverse structural features have been reported. Therefore, many researchers have synthesized these compounds as target structures and evaluated their biological activities. Hydrazides, hydrazones and their adducts have displayed diverse range of biological properties such as potential biological activities [1-6], anti-viral [7-8], anti-tuberculosis [9-10], anti-tumor [11-18], anti-fungal [19-20], anti-convulsant [21], anti-helmintic [22], anti-malarial [23], anti- Inflammatory [24], anti-cancer[25-26], anti-proliferative [27-29], anti-oxidant [30], agricultural agents [31]. Therapeutic protocols for the treatment of HIV infection are mainly based on the combined use of reverse transcriptase, protease, and more recently, of cell fusion and entry inhibitors. Although drugs targeting reverse transcriptase and protease are in wide use and have shown effectiveness, the rapid emergence of resistant variants, often cross-resistant to the members of a given class, limits the efficacy of existing antiretroviral drugs. Therefore, it is critical to develop new agents directed against alternate sites in the viral life cycle. Moreover, many selectively chloro-substituted organic compounds show peculiar pharmacological and agrochemical properties. The work reported herein was aimed at the preparation of some new thiophenoladducts of acidhydrazones with anticipated biological activities.
Materials and methods
Experimental
Anhydrous solvents and all reagents were purchased from, Sigma-Aldrich, B.D.H., Excel-R, Extra pure E. Merk quality, Acros or Carlo Erba. Reactions involving air- or moisture-sensitive compounds were performed under a nitrogen atmosphere using oven-dried glassware and syringes to transfer solutions. Melting points (m.p.) were determined using an electrothermal melting point or a Köfler apparatus and are uncorrected. Infrared (IR) spectra were recorded as thin films or nujol mulls on KBr plates with a Perkin-Elmer-781 IR or 983 -Spectrophotometer and are expressed in ν (cm-1). Nuclear magnetic resonance spectra (1H-NMR) was determined in DMSO and recorded on a Varian XL-200 (200 MHz) or a Varian VXR-300 (300 MHz). Chemical shifts (δ scale) are reported in parts per million (ppm) downfield from tetramethylsilane (TMS) used as internal standard. Splitting patterns are designated as follows: s, singlet; d, doublet; t, triplet; q, quadruplet; m, multiplet; brs, broad singlet; dd, double doublet. The assignment of exchangeable protons (-OH and -NH) was confirmed by addition of D2O. Analytical thin-layer chromatography (TLC) was carried out on Merck silica gel, F-254 plates. For flash chromatography Merck Silica gel-60 was used as stationary phase with a particle size 0.040-0.063 mm (230-400 mesh ASTM). Elemental analyses were performed on a Perkin-Elmer-2400 spectrometer, and were within ±0.5% of the theoretical values.
Synthesis of Ethyl-2-(2, 5-dichloroanilido) ethanoate [1]
A mixture of 2, 5-dichloroaniline (10ml) and diethylmalonate (20ml) was refluxed for forty five minutes in a round bottomed flask fitted with an air condenser of such a length (14″) that ethanol formed escaped and diethylmalonate flowed back into the flask. Contents were cooled, ethanol (30 ml) was added, when malon-2, 5-dichlorodianilide separated out. It was filtered under suction. The filtrate was poured on to crushed ice (Ca160g) and stirred when ethyl-2-(2, 5-dichloroanilido) ethanoate precipitated as green mass. On recrystallization from aqueous ethanol (50%), ester was obtained as white crystals. Yield: 83%, M. P.: 900C, M. W.: 276. Anal. Calculation for C11 H11 N1 O3 Cl2: Found: C 47.7, H: 4.0, O: 17.2, N: 5.1, Cl: 25.4, Calcd. C: 47.8, H: 4.0, O: 17.4, N: 5.1, Cl: 25.7. IR [KBr] Vmax Cm-1 : 1665-1660 [C=O diketone], 1290 [-O- Ester], 760-755 [2,5-disubstituted benzene], 1090 [C-Cl Stretching], 1590, 1520 , 1440 [C=C ring stretching], 3150 [N-H Stretching], 3040[C-H aromatic], 1330-1320 [C-H Stretching]. PMR (DMSO): δ 4.45 (2H, s, CO-CH2-CO), 4.0 (2H, s, NH2), 7.4-8.7 (3H, m, Ar-H), 9.4 (1H, s, CO-NH D2O exchangeable), 10.4 [1H, s, Ar-NH D2O exchangeable].
Synthesis of Ethyl-2-[(N-acetyl) 2, 5- dichloroanilido] ethanoate [2]
Acetyl chloride (4.74 gm; 0.06 mol), dioxane (6 ml), Ethyl-2-(2, 5-dichloroanilido) ethanoate (16.56 gm; 0.06 mol) and triethylamine (5.7 gm; 0.06 mol) were placed in a round bottomed flask carrying reflux condenser having calcium chloride guard tube. The contents were heated on a boiling water bath for two hours and kept over night when triethylamine hydrochloride separated. It was filtered under suction and the filtrate was poured on to crushed ice (Ca180 g) and stirred when ethyl-2-[(N-acetyl) 2, 5-dichloroanilido] ethanoate separated or solid. It was filtered under suction, dried and purified by recrystallization from aqueous methanol (1:1) in white crystals. Yield = 77 %, MP = 96°C Analytical calculation for C13 H13 O4 N1 Cl2 : [FW = 318 ] , Calculated: N 02.95 , C 45.64, H 03.38 , O 13.50 , Cl 15.00 , Found : N 02.94, C 45.62 , H 03.37 , O 13.52 , Cl 15.02. IR [KBr] Vmax cm-1 : 1720 [ C=O diketone ], 1310 [ -C-O- Ester], 765 [ 2,5- disubstituted benzene ], 1095 [ C-Cl Stretching ], 1590, 1525 , 1440 [C=C Ring stretching ], 3160 [N-H Stretching], 3040[C-H aromatic], 1330-1325 [C-H Stretching ]. PMR (DMSO): δ 4.42 [2H, s, CO-CH2-CO], 4.1 [2H, s, NH2], 7.2-8.5 [3H, m, Ar-H], 9.5 [1H, s, CO-NH D2O exchangeable], 10.8 [1H, s, Ar-NH D2O exchangeable].
Synthesis of 2-[(N-acetyl) 2, 5- dichloroanilido] acetohydrazide [3]
Ethyl-2-[(N-acetyl) 2, 5-dichloroanilido] ethanoate (9.54 gm; 0.03 mol), ethanol (10 ml) and hydrazine hydrate (15 ml; 80%) were mixed together and stirred for thirty five minutes. 2-[(N-acetyl) 2, 5-dichloroanilido] acetohydrazide was filtered under suction and recrystallised from ethanol in white crystals. Yield; 74%, MP = 178°C, MW 304: Analytical calculation for C11 H11 N3 O3 Cl2 : Calculated ; N 09.04 ,C 41.32 ,H 03.01 ,O 10.33, Cl 15.28, Found; N 09.01, C 41.30, H 03.00, O 10.31, Cl 15.27 . IR [KBr] Vmax cm-1: 3165 [N-H Stretching], 3050 [C-H aromatic], 1670 [C=O diketone], 1430 [C-Cl aromatic], 1595, 1520, 1445 [C=C ring stretching]. PMR (DMSO): δ 4.44 (2H, s, CO-CH2-CO), 4.4 (2H, s, NH2), 7.3-8.5 (3H, m, Ar-H), 9.5 (1H, s, CO-NH D2O exchangeable), 10.5 (1H, s, Ar-NH D2O exchangeable).
Synthesis of 2-[(N-acetyl) 2, 5-dichloroanilido] acetohydrazones [4]
2-[(N-acetyl) 2, 5-dichloroanilido] acetohydrazide (0.001 mol) and (0.001 mol) of aromatic aldehyde or ketone [such as benzaldehyde] dissolve in absolute alcohol and added 2-drops of conc. H2SO4 and stirred for 25 minutes. It was filtered under suction and recrystallised from hot ethanol. Color: Silver white, Yield: 86%, M.P= 218 0C, F.W: 392, Analytical calculation for C18H15O3N3Cl2, Calculated: N 12.04, C 54.85, H 03.71, O 09.14, Cl 20.28, Found: N 11.98, C 54.82, H 03.70, O 10.31, Cl 20.26. IR Absorption band (cm–1): 3160 (N–H stretching), 2960–2975 (C–H aliphatic), 1665–1660 (C=O Ketone), 795–780 (C–Cl Stretching), 765 (2, 5-disubstituted benzene). NMR Spectra: (d DMSO), 2.20(2 H, s, CH2), 4.22(1 H, s, NH), 6.96–7.2 (10 H, m, ArH. Synthetic strategy has been out lined in scheme-I. Mechanism for the formation of acid hydrazones is given in chart-I.
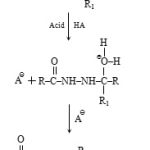 |
Chat 1:
|
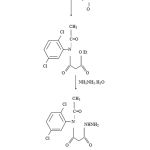 |
Scheme 1:
|
 |
Table 1:
|
Biological evaluation
Anti-bacterial activity
Newly synthesized thiophenoladducts of acidhydrazones were screened for their anti-bacterial activity against the gram positive bacteria S. albus, S. aureus and gram negative bacteria E.coli and Pseudomonas piosineus by agar plate disc diffusion method at 30 μg/mL concentration. Ampicillin and tetracycline were used as a reference compounds. The compound (1, 5, 15, 16) shown significant activities and compound (2, 4, 9, 12, 14) have shown moderate activity.
Anti-fungal activity
The same compounds were tested for their antifungal activity against Candida albicans, Aspergillus niger and Alternaria alternata at concentration of 30 mg/ml using Savored dextrose agar media. The compound (3, 8, 11, 13) shown significant activity and compound (1, 7, 10, 17) have shown moderate activity against Candida albicans and Aspergillus niger. All the other compounds did not show significant activity against the fungi at the concentration used.
Results and discussion
Thiophenoladducts of various acidhydrazones have been synthesized by the reaction of 2-[N- (acetyl) 2, 5-dichloroanilido] acetohydrazide with various Carbonyl Compounds in 44 to 69% yield. Hydrazone-thiophenoladducts are white, brown and yellow colour solids, having high melting points. The structure of all the compounds are confirmed by IR, NMR, and Mass spectral data and are further supported by correct elemental analysis. Newly synthesized compounds have been tested for their antibacterial activity against gram positive bacteria S. albus, S. aureus and gram negative bacteria E.coli and Pseudomonas piosineus. The compound (1, 5, 15, 16) shown significant activities and compound (2, 4, 9, 12, 14) have shown moderate activity. The same compounds were tested for their antifungal activity against Candida albicans, Aspergillus niger and Alternaria alternata at concentration of 30 mg/mL using savored dextrose agar media. The compound (3, 8, 11, 13) shown significant activities and compound (1, 7, 10, 17) have shown moderate activity against Candida albicans and Aspergillus niger. All the other compounds did not show significant activity against the fungi at the concentration used.
Conclusions
Newly synthesized compounds have been tested for their antibacterial activity against gram positive bacteria S. albus, S. aureus and gram negative bacteria E.coli and Pseudomonas piosineus by agar plate disc diffusion method at 30 μg/mL concentration. Ampicillin and tetracycline were used as a reference compounds. The compound (1, 5, 15, 16) shown significant activities and compound (2, 4, 9, 12, 14) have shown moderate activity. The same compounds were tested for their antifungal activity against Candida albicans, Aspergillus niger and Alternaria alternata at concentration of 30 albicans and Aspergillus niger. All the other compounds did not show significant activity mg/mL using Savored dextrose agar media. The compound (3, 8, 11, 13) shown significant activities and compound (1, 7, 10, 17) have shown moderate activity against Candida against the fungi at the concentration used.
Acknowledgements
The authors are thankful to Director, C.D.R.I. Lucknow, for elemental analysis, Director, D.R.D.E. Gwalior, for spectral studies, and Director, Cancer Hospital and Research Institute, G.R. Medical College and Birla Institute of Medical Research, Gwalior, for biological activities. We are also grateful to principal SMS Government Model Science College, Gwalior, for providing research facilities.
References
- Rahman, V.M.; Mukhtar, S.; Ansari, W.H.; Lemiere, G. ; J. Med. Chem. 2005, 40, 173 – 184.
- Dimmock, J.R.; Vashishtha, S.C.; Stables, J.P. ; J. Med. Chem. 2000, 35, 241-248.
- Yapia, R.; La Mara, M.P.; Massieu, G.H.; Biochem. Pharmacol. 1967, 16, 1211-1218.
- Sava, G.; Perissin, L.; Lassiani, L.; Zabucchi, G. ; Biol. Interact., 1985, 53, 37-43.
- Ajani, O.O.; Obafemi, C.A.; Nwinyi, O.C.; Akinpelu,D.A.; Bioorg. Med. Chem. 2010, 18, 214-21.
- Bhagavan, N.V. Medical Biochemistry; Elsevier Science B.V.: Amsterdam, the Netherlands, 2002; Volume 17, pp. 331-363.
- Saulnier, M.G.; Velaprthi, U.; Zimmermann, K. In Progress In Heterocyclic Synthesis; Gribble, G., Ed.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2005; Volume 16, pp. 228-271.
- Faroumadi, A.; Kiano, Z.; Soltani, F.; Farmaco 2003, 58, 1073-1076.
- Holdiness, M.R.; Tubercle 1987, 68, 301-309.
- Short, E.I.; Tubercle 1962, 43, 33-42.
- Bokharev, V.V.; Ghidaspov, A.A.; Peresedova, E.V.; Heterocycl. Comp. 2006, 42, 1096-1106.
- Fernando, R.P.; Maia, P.I.; Leite, S.R.; Deflon, V.M.; Batista, A.A.; Sato, D.N.; Franzblau, S.G.; Leite C.Q. ; J. Med. Chem. 2010, 45, 1898-1905.
- Brzozowski, Z; Czewski, F.S. ; J. Med. Chem. 2002, 37, 709-720.
- Sherif, A., Rostom, F.; Bioorg. Med. Chem. 2010, 18, 2767-2776.
- Mohareb, R.M.; Mohamed A.A.; Molecules, 2010, 15, 3602-3617.
- Mohareb, R.M.; El-Arab, E.E.; El-Sharkaway, K.A.; Pharm. 2009, 77, 355-365.
- Hoda Z. Shams, Rafat M. Mohareb, Maher H. Helal ;Amira E. Mahmoud ; Molecules 2011, 16, 52-73.
- Rafat M. Mohareb; Daisy H. Fleita; Ola K. Sakka; Molecules 2011, 16, 16-27.
- Wardakhan, W.W.; Shams. H.Z.; Moustafa, H.E.; Phosph. Sulf. Silicon 2005, 180, 1815-1827.
- Pinto, E.; Queiroz, M.-J.R.P.; Vale-Silva, L.A.; Oliveira, J.F.; Begouin, A.; Begouin, J.-M.; Kirsch, G.; Chem. 2008, 16, 8172-8177.
- Vicini, P.; Zani, F.; Cozzini, P.; Doytchinova, I. ; J. Med. Chem. 2002, 37, 553-564.
- Isabel C.F.R., Ricardo C. C., Letícia M. E., Maria-João R.P.Q.; Bioorg. Med. Chem. Lett. 2004, 14, 5831-5833.
- Melnyk, P.; Leroux, V.; Serghergert, C.; Grellier, P. Design, Bioorg. Med. Chem. Lett. 2006, 16, 31-35.
- Dannhardt, G.; Kiefer, W.; Krämer, G.; Maehrlein, S.; Nowe, U.; Fiebich, B. ; J. Med. Chem. 2000, 35, 499-510.
- Zheng, L.W.; Wu, L.L.; Zhao, B.X.; Dong, W.L.; Miao, Y.J. ; Med. Chem. 2009, 17, 1957-1962.
- Xia, Y.L; Chuan-Dong, F.; Zhao, B.X.; Zhao, J.; Shin, D.S.; Miaom J.Y. ; J. Med. Chem. 2008, 43, 2347-2353.
- Brault, L.; Migianu, E.; Néguesque, A.; Battaglia, E.; Bagrel, D.; Kirsch, G. ; J. Med. Chem. 2005, 40, 757-763.
- Starčević, K.; Karminski-Zamola, G.; Piantanida, I.; Zinić, M.; Sǔman, L.; Kralj, M. ; Am. Chem. Soc. 2005,127, 1074-1075.
- Romagnoli, R.; Baraldi, P.G.; Carrion, M.D.; Cara, C.L.; Cruz-Lopez, O.; Preti, D.; Tolomeo, M.; Grimaudo, S.; Cristina, A.D.; Zonta, N.; Balzarini, J.; Brancale, A.; Sarkar, T.; Hamel, E. Design, Med. Chem. 2008, 16, 5367-5376.
- Ferreira, I.C.F.R.; Queiroz, M.R.P.; Vilas-Boas, M.; Estevinho, L. M.; Begouin, A.; Kirsch G. ; Med. Chem. Lett. 2006, 16, 1384-1387.
- Mamolo, M.G.; Falagiani, V.; Zampieri, D.; Vio, L.; Banfo, E.; IIFARMACO 2001, 56, 587-592.







