Prabhat Ranjan
Department of Zoology, S.M.D. Degree College, Jalalpur (India).
Abstract
The ovarian cycle and spawning season of Ophiocephalus punctatus from Chapra, BIHAR, a subtropical area, is described. The oocyte shows six well differentiated stages of maturation and the fish has a prolonged spawning season extending from May to August. The spawning tends to be bimodal in character. The stages iii the formation of the yolk nucleus of Balbiani are described and the probable origin of this cytoplasmic inclusion is discussed.
Keywords
Ovarian cycle; spawning season; Ophiocephalus punctatus
Download this article as:| Copy the following to cite this article: Ranjan P. Ovarian Cycle and Spawning Season of Ophiocephalus punctatus, Inhabiting Chapra Waters, Bihar. Biomed Pharmacol J 2011;4(1) |
| Copy the following to cite this URL: Ranjan P. Ovarian Cycle and Spawning Season of Ophiocephalus punctatus, Inhabiting Chapra Waters, Bihar. Biomed Pharmacol J 2011;4(1). Available from: http://biomedpharmajournal.org/?p=1752 |
Introduction
Ophiocephcthes punclatus Bloch is a widely distributed and economically important freshwater fish capable of adapting itself to diverse climatic conditions over nearly half of the world (Nelson, 1976). Successful exploitation of any fish solicits a thorough knowledge of its reproductive biology. In view of the considerable variations reported in gonadal cycle, spawning time and spatial behaviour of this fish (Sunderraj, 1916; Khan, 1924; Mukherjee, 1945; Swarup, 1954; Belsare, 1962), a study was undertaken to find Out the exact pattern of ovarian cycle and spawning season of 0. punctatus in Jammu, a sub-tropical province of J & K state, India. Jammu is located at an altitude of 300 meters between 32°15’ to 33°30’N latitude and 740 to 76°l5’E longitude.
Material and methods
Weekly collections of Ophiocephalus punctatus were made from freshwater ponds in the area, their ovaries removed and fixed in dichromateformalin-acetic acid fixative within two hours of the catch. After routine paraffin embedding, 5~8μ thick sections were cut and stained with Mallory’s triple stain for microscopical studies. Stages of maturity of ova were fixed after the method of Srivastava and Rathi (1970). The dimetrical determinations were made of every 10th section and average worked out from 50 such measurements. The different stages of maturing eggs were translated into percental values. Weight of fish and ovaries were determined and gonado-somatic index (GSI) calculated by the formula: weight of ovary ÷ weight of fish ´ 100 and the results thus obtained were recorded in Table I.
Observations
General morphology and histology. The ovaries are paired and when young are transluscent structures which turn yellow upon maturing. These lie addpressed to the dorsal body wall closely flanking the air-bladder within their inner margins. Posteriorly each ovary is continuous with its own oviduct. The oviducts from two sides meet near termination to open to the outside by a common aperture.
Each ovary remains ensheathed by a tough covering, the tLlnica albuginea which is lined by
single layered germinal epithelium. The tunica albuginea is made up of muscles and connective tissue. The tunic together with the germinal
Table 1. Seasonal variations in the average diameters of large oocytes and their nuclei and the goriado-somatic index of the fish. N=50.
| Month | Average
diameter of oocyte (μ) |
Average
diameter of oocyte (μ) |
Gonadosomatic
index of fish |
| January | 240 | 112 | 0.306 |
| February | 288 | 112 | 0.414 |
| March | 480 | 160 | 1.200 |
| April | 720 | 128 | 4.333 |
| May | 560 | 144 | 2.247 |
| June | 950 | 160 | 4.604 |
| July | 720 | 144 | 3.352 |
| August | 690 | 128 | 2.445 |
| September | 480 | 112 | 1.108 |
| October | 160 | 80 | 0.411 |
| November | 192 | 80 | 0.421 |
| December | 208 | 96 | 0.280 |
epithelium projects at places into ovocoel to form the characteristic ovigerous lamellae (Fig.5). Embedded in the ovigerous lamellae are observed the oogonia and oocytes at various stages of development which do not conform to any regular arrangement. A discontinuous mass of filling tissue, the stroma (Fig. 3) is seen to lie between oocytes and with in the ovocoel ofthe ovigerousl aniellae. The germinal epithelium lining the ovigerous lamellae shows extensive prolificity in the production of oocytes from October to March with peak activity observed in February and March (Figs. 2, 5). This activity involves the transformation of some of the germinal cells initially into oogonia from which eventually arise the future oocytes. Supplements to this crop of oocytes are also made from oogonial nests (Fig. I) and the resting ocgonia from previous year’s crop (Figs. 7, II).
Interstitial tissue appears as isolated patches of irregular cells during the period extending from October to early February (Fig. 6). These cells often stain differentially and may appear as yellow or brown in Mallory’s triple stain. Soon after February they become scarce almost to the point of disappearance in the ovaries.
Changing morphology of growing oocytes. The entire sequence of events that a growing oocyte in this fish passes through before getting transformed into an ovum can be conveniently arranged in the following six stages:
Stage I: Chromatin-nucleolar stage (Fig. 2). The first recognizably differentiated oocytes are placed in this stage and vary in size from 16 to 98/1. Their nuclei range in diameter from 12 to
50 . The nuclei are centrally placed and each bears an eccentric primary nucleolus which measures from 4 to 121L in diameter (Fig. 3). However, a few oocytes were seen to contain several deeply staining nucleoli, much smaller in size. A thin follicle layer is seen to surround the oocytes.
Stage 2: Peri-nucleolar stage. The oocytes in this stage are surrounded by a flattened follicular layer. The size of the oocytes, however, varies from 100 to 200 with nuclei varying from 50 to 100g. A number of morphological changes are characteristic of these oocytes: (i) the well sized primary nucleolus characteristic of stage I oocytes becomes indistinguishable from a number of small fuchsinophilic nucleoli arranged penipherally in the nuclei of nearly 98% of ocyteso at this stage; (ii) a well differentiated darkly staining body, the yolk nucleus of Balbiani (Fig. 4), about 12μ in diameter makes its appearance in the ooplasm close to the nucleus; (iii) cytoplasm shows a regional differentiation into a lightly staining peripheral and a darkly staining pen-nuclear zone (Fig. 5); and (iv) a number of oocytes, mostly of higher diameters, show an altered position of yolk nucleus of Balbiani from the neighbourhood of the nucleus to close proximity of the periphery of the oocytes (Fig. 5).
Stage 3: Yolk vesicle stage. The oocytes included in this stage measure from 200 to 300μ in diameter with nuclei measuring 100~160μ in diameter, In oocytes of this slage. a distinct vacuolated zone nearly 8μ deep is observed in the ooplasm all along the periphery (Fig. 7). These vacuoles are the yolk vesicles. The yolk nucleus of Balbiani persists (Fig. 8). The nuclear membrane assumes an irregular outline and several nucleoli are seen pushed into its cranulations. A few nucleoli were observed to have even extruded out into the ooplasm but not very far from the nucleus. The follicular epithelium thickens and a thin vitelline membrane forms around the ooplasm of the oocyte.
Stage 4: Vitellogenetic stage (Fig. 9). During this stage, oocytes varying in diameter from 300 to 650μ with apparently no increase in the size of nuclei are included. The yolk globules are seen to deposit in the yolk vesicles whose number increases with the increase in the size of the oocytes. The yolk globules were also seen to be deposited outside the vesicles. The extrusion of nucleoli from the nucleus into the ooplasm is generally speeded up. The vitelline membrane is prominent. A clear and distinct theca is observed around a prominent follicular epithelium (Fig. 10).
Stage 5: Maturation stage (Fig. 11). The maturing oocytes measure from 650 to 950 μ in diameter and are characterized by the absence of a distinguishable nuclear membrane, Yolk deposition is apace. The typical three layers of a mature ovum are distinct and prominent.
Stage 6: Ripe egg stage. The eggs at 950 μ diameter, regarded as ripe eggs, are opaque. spherical and bear comparatively thin egg membranes which do not show a specific adhesive
nature
Annual cycle of the oocytes. Fig. 13 represents the seasonal growth pattern of an oocyte together with its percental contribution in the general make up of the ovary. A perusal of the above figure reveals that stage 1 and 2 oocytes are found in the ovary throughout the year although in varying numbers. These oocytes are maximally found in the winter months i.e., from October to January reaching a peak in their count in November and December when the average water temperature of the pond is around 14°C. Stage 3 oocytes remain comparatively low until their number increases over the period and a maximal count (35%) attained in February. The numerical increase in stage 3 oocytes corresponds well with progressive increase in the water temperature from January onward. Their peak occurrence (stage 3) is followed by the appearance of stage 4 oocytes, the latter showing two peaks, one in March contributing 41% and other in May contributing 24% of the total ovular crop in the ovary. The stage 4 oocytes in turn alternate with the peaks of stage 5 and 6 oocytes in April (69%) and June (72%) during which months the stage 4 oocytes are least available in the ovary.
Discussion
Ophiocephalus punctatus has a cystovarian ovary and the ova are led out of the body through a short oviduct Histologically, a spent maturing ovary contains large number of germ cells at different stages of maturation. The germ cells get refurbished from the lamellar epithelium, residual oogonia and oogonial nests (Figs. I, II), as is true of the species from more tropical areas (Belsare, 1962). This mode of germ cell formation, thus, appears to be a genetically fixed trait for the species with apparently no impact on its ecological diversities. However, in the involvement of oogonial nests for ova production, the species shows similarity with Mugil cephalus (St renger, 1959), and Plecoglossus aft/tells (Honma. 1961).
A lot of confusion exists in the recognition of different stages of a growing fish oocyte as labelled by various workers (Wood, 1930 for Scottish herring shoals; James, 1946 for Lepomis macrochirus and Huro salmoides; Yamamot o, 1956 for Liopsetta obscura: Lal, 1963 for Cirrhna mrigala). In the presently investigated fish, Ophiocephalus punctatus, the stages given for Heteropneustus fossills by Srivastava and Rathi (1970) are readily identifiable xcept for an overlap of yolk granule stage and yolk globule stage (Srivastava and Rathi, 1970) which have, therefore, been merged into one and designated here as the vitellogenetic stage.
During its early growth phase, a young oocyte in Ophiocephalus punctatus shows some changes such as (i) the appearance of primary nucleolus in the nucleus of freshly formed oocyte (Fig. 3), (ii) concomitant increase in the size of nucleus and the attendant oocyte, and (iii) almost concurrent disappearance of primary nucleolus and appearance of yolk nucleus of Balbiani in the ooplasrn (Fig. 4). Considerable doubt exists about the exact origin of yolk nucleus of Balbiani in fishes. Some workers assign its origin to nuclear activity (Hubbard, 1894; Wheelar, 1924; Subramaniun, and Aiyar 1935; Sathyanasen. 1959) and others believe it to be cytoplasmic in origin (Narain, 1951; Chaudhry, 1952; Bara 1960; Nayyar, 1964).
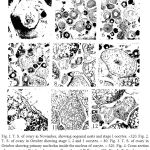 |
Figure 1-12: 1I. T. S. of ovary in November, showing oogonial nests and stage I oocytes.
|
Fig. I. T. S. of ovary in November, showing oogonial nests and stage I oocytes. ´320. Fig. 2. T. S. of ovary in October showing stage 1, 2 and 3 oocytes. ´ 80. Fig. 3. T. S. of ovary in October showing primary nucleolus inside the nucleus of oocyte. ´ 320. Fig. 4. Cross section of an oocyte in October showing yolk nucleus of Balbiani. ´320. Fig. 5. T. S. of ovary in December showing ovigerous larnellae, zonation of cytoplasm and peripheral position of yolk nucleus of Balbiani. ´80. Fig. 6. T. S. of ovary in December showing a patch of interstitial tissue. ´80. Fig. 7. T. S. of ovary in February showing stage 3 oocyte. ´80. Fig. 8. T. S. of ovary in May showing yolk nucleus of Balbiani in stage 3 oocytes ´80. Fig. 9. T. S. of ovary in May showing stage 4 oocytes. ´80. Fig. 10. Crossseciionofan oocyte showing egg membranes. ´480. Fig. 11. T. S. of ovary in June showing stage 5 oocytes. ´32. Fig. 12. T. S. of ovary in July showing airetic oocytes. ´80. AO, atretic oocyte; CZ, cytoplasmic zonation; F, follicular epithelium; IT, interstitial tissue; N, nucleus; OL, ovigerous lamella; ON, oogonial nests; PN, primary nucleolus; RO, residual oogonia; SN, secondary nucleoli; T, theca; V, vitelline; Y, yolk; YNB, yolk nucleus of Batbiani; YV, yolk vesicles.
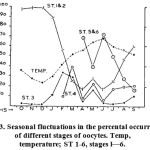 |
Fig. 13. Seasonal fluctuations in the percental occurrence of different stages of oocytes. Temp, temperature; ST 1-6, stages l—6.
|
Appearance of yolk nucleus of Balbiani in Ophiocephalus punctatus oocyte closely following the disappearance of primary nucleolus is fairly suggestive of its nuclear origin and not cytoplasmic. Indirect evidence to this presumption also comes from such facts as (i) similarity in the size of newly appeared yolk nucleus of Balbiani and last observed primary nucleolus in the nucleus and (ii) similarity in the tinctorial behaviour of the two structures (both being fuchsinophilic). The appearance of yolk nucleus of Balbiani followed immediately by cytoplasmic zonation in the oocytes, however, indicates the role the former possibly plays in the maturation of the oocytes in O. punclatus. This view is more or less consistent with that of Belsare (1962) and Bhargava (1971).
Ovarian activity of 0. punctafus follows a cyclic pattern which can be divided broadly into four phases viz., resting phase (from November to January), pre-spawning phase (from February to April), spawning phase (from May to August) and post-spawning phase (from September to October). During the resting phase the oocytes are immature and do not show any yolk deposition (Fig. 1). During the pre-spawning phase there is active vitellogenesis and some of the oocytes are seen on way to maturation (Fig. 7). These precociously mature ova constitute the crop for the first act of spawning. Remaining advanced oocytes mature and are subsequently extruded during the second spawning act being separated from the first by a short interval. During the post-spawning phase any mature ova left unspawned get finally resorbed (Fig. 12).
Variability in the spawning period of this fish from place to place is known. It spawns twice a year at Madras, once in January/February and second time in July/August (Sunderraj, 1916). In Panjab it spawns from late April to the end of July (Khan, 1924). In Bengal, the spawning period extends from June to August (Mukherjee, 1945) and in Madhya Pradesh from May to the end of September (Swarup, 1954). Our investigations on the spawning and breeding in Ophiocephalus punctatus from a sub-tropical area to some extent agrees with the reports of Swarup (1954) and Khan (1924). A look at the graphic representation of stages (Fig. 13) and gonado-somatic index (Table I) reveals that in Jammu waters this fish has a prolonged spawning period extending from May to the end of August. The spawning tends to assume a bimodal character with stage 5 and 6 laiden ovaries appearing in late April and again in June. A dip in the ovular number in between these two months is suggestive of an act of spawning but stands in sharp contrast to observations of Nair (1958) on Hi/isa ilisha, where the fish gonads attain two peaks in a year of which the first peak results in atresia and subsequent resorption of the follicles rather than culminating in a spawning act. How- ever, as the temperature keeps on rising to a maximum of 34°C in June the ovular number (stage 5 & 6) correspondingly rises whereafter not only the temperature of the surrounding water but the stage 5 & 6 ova also show a gradual decline. The progressive decrease in number of these ova from July onward in fact represents the normal act of spawning.
Seemingly this bimodal spawning tendency could be an adaptation to a subtropical habitat of this species in a bid to accomplish a temperate nature by increasing the number of its spawning acts, a feature which is already reported in an allied species from China (Chen and Lin. 1935). It may thus be concluded that the two spawning seasons known for Ophiocephalus punctaws from more tropical areas are merged into a single prolonged spawning season with multiplicity of spawning acts, which characters this fish, as it moves to more temperate climate.
References
- Bara, G. 1960. Histological and cytological changes in the ovaries of mackerel Scoinber sconther L. during the annual cycle. Rev. Fac. Sci. Univ., 25: 4l—9t.
- Belsare, D. K. 1962. Seasonal changes in the ovaries of Ophiocepliolus punctalus Bloch. tnd. J. Fish., 90): 140– 154.
- Bhargava, H. N. 1971. Yolk nucleus of Balbiani in Indian freshwater goby, Glossogobius giurus. Curr. Sci., 40(21): 575–577.
- Chaudhry, H. 5. 1952. The yolk nucleus of Balbiani in teleostean fishes. Zeit. für Zellforsch, 37, 5: 455 — 466.
- Honma, Y. 1961. Studies on the endocrine gland of salmoids fish, ayu, Plecoglossus altivelis Temminek et Schlegel. IV. The fate of unspawned eggs and new crops of oocytes in spent ovary. Bull. Japan. Soc. Sci. Fish., 27: 837–480.
- Hubbard, J. W. 1894. The yolk nucleus of Baibiani in Cymatogaster aggregatus gibbons. Proc. Amer. Phil. Soc., 33. (Not seen in original).
- James, M. F. 1946. Histology of gonadal changes in the blue gill Lepomis macrochirus Rafinesque and the large mouth bass Huro solmolds Lacepede. J. Morph,, 79: 63—92.
- Khan, H. 1924. Observation on breeding habits of some freshwater fishes in the Panjab. J. Bom. Nat. Hist. Soc., XXIX L 958.
- Lal, M. B. 1963. Morphological and cytological studies of oocytes of Cirrl,ino mrigaln (Hamilton) with particular reference to lipod. Proc. Nat. Inst. Sd. India, 29: 585—60l.
- Mukherjee, H. K. 1945. Life histories of some carnivorous fishes of Bengal. Sci. & Cult., II: l02— 103.
- Nair, P. V. 1958. Seasonal changes in the gonads of Hilso ilishia Hamilton. Philipp. J. Sci., 87 (3): 255-’ 276.
- Narain, D. 1951. Proc. Nat. Acad. Sci. India..
- Nayyar, R. P. 1964. Quart. J. Microsco. Sci., 105.
- Nelson, J. S. 1976. The fishes of the world. Jhon Wiley & Sons, New York, xiii+4l6 pp.
- Sathyanesen, A. H. 1959. Natureswissenschaften, 46.
- Srivastava, P. N., and S. K. Rathi. 1970. Effect of radiation on reproductive system of indian catfish Heteropneustus fossi/is Bloch. 1. Annual cycle in development of ovarian eggs. Acta Anal,, 74: 114- 125.
- Strenger, A. H. 1959. A study of structure and development of reproductive tissue of Mugilcephalus L. Zoologica, 44: 53-.-70.
- Sunderraj, B. 1916. Notes on freshwater fishes of Madras. Rec. md. Mus., 12.
- Subramanium, M. K., and R. G. Aiyar. 1935. Oogenesis of Acenfrogobius nettle with special reference to the behaviour of nucleoli. J. Roy. Micr. Soc., 55.
- Swarup, H. 1954. The development of chondrocranium of Ophiacephalus puactanis. Sang. Univ. J: 61~79.
- Wheeler, J. F. G. 1924. The growth of egg in dab, Pleuronectes limanda. Quart. M. Micr. Sci., 68: 641–660.
- Wood, H. 1930. Scottish hearring shoals. Prespawning and spawning movements. Fish Bd. Sci. Invest., 1: 1.
- Yamamoto, K. 1956. Studies on the formation of fish egg. 1. Annual cycle in the development of ovarian eggs in flounder, Leopsetta obscure. J. Fae. Sci. Hokkaido Univ., series VI, Zool., 12: 362-373.







